Information
Journal Policies
Effects of Microporous Polysaccharide Powder in a Model of Dilution on Viscoelastic Characteristics of Clot Formation - An In-Vitro Study
Lion Sieg1,Hendrik Eismann2,Carsten Schumacher3,Felix Floricke4,Kai Johanning,5 Alexander A.Hanke6
Copyright : © 2017 Authors. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction: Trauma is still a major reason for global morbidity and mortality. Massive bleeding is the major cause for trauma related deaths. One part of bleeding control is the use of topical haemostatic agents in combination with other surgical measures. A novel approach is the use of polysaccharide microbeads which are directly applied onto bleeding areas to initiate hemostasis by building a tight viscous mesh of gel and blood components. Purpose of our study was to determine effects of such a novel polysaccharide (4DryField (4DF)) on viscoelastic coagulation parameters as assessed by rotational thromboelastometry.
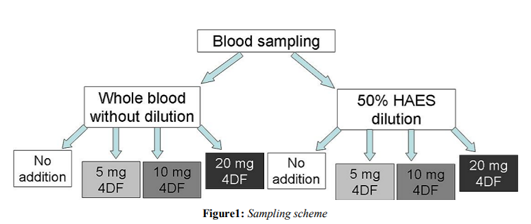
Materials and Methods: Following IRB approval and informed consent blood samples were taken from 10 healthy volunteers. To assess effects of 4DF, rotational thromboelastometry after extrinsically activation was performed either native or with addition of 5 mg, 10 mg or 20 mg 4DF to the test sample volume of 300μl. Recorded parameters were speed of coagulation and clot firmness. Furthermore, to simulate treatment under conditions of dilutional coagulopathy all tests also were performed after 50% HAES dilution.
Results: Application of 4DF significantly increases coagulation speed and clot firmness in both, native blood and in diluted blood. In native blood, coagulation parameters were improved to the upper level of normal range. In 50% HAES diluted blood, coagulation was significantly improved from severely reduced to slightly below normal range.
Conclusion: Application of 4DF improves coagulation even in 50% HAES diluted blood and thus, 4DF might be capable to seal bleeding areas. Further clinical trials are necessary to prove in vivo efficacy.
ROTEM, rotational thromboelastometry, Microporous Polysaccharide Powder, blood coagulation, point of care, Anesthesiology
Trauma is still a major reason for global morbidity and mortality[1]. Massive bleeding is the main cause for trauma related deaths[2] and is considered to be most common preventable[3,4]. More than 30% of bleeding trauma patients present trauma induced coagulopathy (TIC) at hospital admission[5,6]. The current European guideline on management of major bleeding and coagulopathy following trauma recommend a quick and aggressive treatment of bleeding trauma patients. This contents a direct transfer to an appropriate trauma treatment center to establish hemostasis and combined with other surgical measures the use of topical haemostatic agents[7].
Utilization of topical hemostatic agents in severe bleeding has been described since the beginning of the ancient world[8]. Several agents characterized by disparate mechanisms are commercially available. The use of these agents is common in the prehospital as well as in the hospital setting and application of topical haemostatics is well reported[9-11].
One way for classification of topical hemostatic agents is based on the mechanism of action and effects on tissues as specified by Khoshmohabat et. al.[10]. Following groups of agents can be discriminated:
1) Factor concentrators: work through fast absorption of blood water content concentrating the cellular and protein components resulting in clot formation.
2) Mucoadhesive agents: work through a strong adherence to tissues and physically block bleeding from wounds by sealing them
3) Procoagulant supplementors: delivering procoagulant factors to the hemorrhagic wound.
Polysaccharid particles as investigated in this study are classified to group 1.
Application of polysaccharid particles as a hemostatic agent has been shown in clinical and experimental studies in different settings to be effective and save[12].
Poehnert et. al demonstrated that 4DryField® PH (4DF, PlantTec Medical GmbH, Bad Bevensen, Germany) is not cytotoxic, well tolerated in doses of up to 1.09g/kg bodyweight and mainly degraded within days. Biocompatibility of 4DF in vitro and in rat model in vivo was rated excellent[13]. 4DF particles minimize bleeding and form a gel which itself is also highly effective as a barrier against adhesion formation[14,15].
4DF consists of polysaccharide particles which have a high capability to absorb water as described above. However, Korell et al. observed application of 4DF in gynecological patients with large size peritoneal trauma and massive bleeding and described a rapid and sufficient hemostasis. After application of 4DF there was neither necessity for other hemostatic agents, nor for conversion from laparoscopic to open surgery. Also postoperative transfusions were not necessary15,16. As possible reason, a concentration effect of coagulation factors and blood cells in wounds to accelerate clotting enhancing hemostasis is discussed[16,17].
Current evidence suggest that excessive resuscitation with i.v. fluids before bleeding control in active bleeding trauma patients can increase bleeding[18] and worsen TIC[19]. There are still trauma patients receiving 2-4l of fluid prehospitally and/or during initial resuscitation[20,21]. Main reason for this iatrogenic dilution is unguided administration of fluids in the acute phase of trauma2. It is known that bleeding trauma patients who initially received more than 2 liters of fluid for resuscitation compared to a low volume group had a significantly worse coagulation profile, required more blood products and had higher incidence of organ failure[22]. Thus, extended prehospital fluid therapy in severely injured trauma patients can be considered as an independent risk factor for mortality[23].
Aim of the present study was investigation of the impact of 4DF on dynamic viscoelastic coagulation parameters as assessed by rotational thromoelastometry with a focus on possible dose dependent effects of 4DF in undiluted as well as in 50% HAES diluted whole blood samples.
2. Materials And Methods
The study was approved by the local ethics committee. 10 participants were included into the study. All participants gave informed oral and written consent, were healthy at the time and had no medication intake for at least seven days.
Blood samples were drawn form a peripheral vein of the upper extremity after appropriate and standardized venipuncture. Samples were collected into citrated tubes (Sarstedt AG & Co, Nümbrecht, Germany) and the first 5ml of blood were discarded and remaining tubes were filled with the recommended volume of blood.
5 mg, 10 mg or 20 mg of 4DryField® PH (4DF, PlantTec Medical GmbH, Bad Bevensen, Germany) was added to each test into the test-cup before test initiation (sample volume 300μl). Then extrinsically activated tests (EXTEM) were performed according to manufacturer´s standard procedure advice.
For evaluation of dilutional effects all tests were also performed in a 1:1 dilution with 6% hydroxyethystarch (HAES 6%). The specific protocol of sample preparation is shown in figure 1.
All tests were performed on a commercially available rotational thromboelastometer (ROTEM delta; TEM International GmbH, Munich, Germany) and were initiated without further delay. Principle and procedure of ROTEM as viscoelastic coagulation-testing device is similar to classical thrombelastography as described by H. Hartert24 and described in various publication before[25,26]. In brief, clot formation alteration is measured by the device and displayed in typical tracings as well as different parameters of time and clot firmness.
We performed an extrinsically activated test (EXTEM)[27] which analyzes the coagulation process after activation by recombinant tissue factor. Following parameters were recorded:
Coagulation Time (CT (s)) which describes time from start of the reaction until first clot formation. Furthermore, Clot Firmness after 10min (A10 (mm)), 20min (A20(mm)), and the Maximal Clot Firmness (MCF (mm)) of the entire measurement were registered.
Data were exported to Prism 6 (GraphPad Prism version 6.0, GraphPad Software, La Jolla, California USA) for further descriptive and explorative data analysis. All data are expressed as mean values ± standard deviation.
Analysis of variance (ANOVA) for repeated measures was used after testing on normal distribution (Kolmogorow-Smirnow-test). A p-value < 0.05 was considered as significant.
3. Results
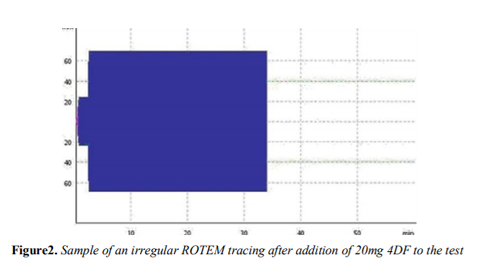
The 20 mg 4DF group (which is comparable to recommended normal dosage) all showed irregular rotation thromboelastometry tracings. Thus, results were considered to be irregular and were eliminated from statistical analysis. An example of an irregular rotation thromboelastometry tracing in the 20mg 4DF group is shown in figure 2.
Further results are shown in tables 1 and 2 as well as in figures 3 and 4.
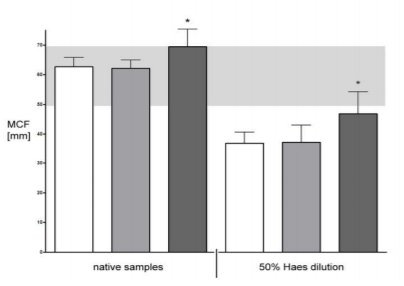
Addition of 10mg 4DF significantly reduces CT and increases A10, A20 significantly in native blood as it does in diluted blood. MCF was also affected. Clot firmness parameters were significantly improved in both undiluted as well as in diluted samples.
In native blood coagulation parameters were improved to the upper level of normal range. In 50% HAES diluted blood EXTEM values were improved from severely reduced to slightly below normal range.
4. Discussion
Our results suggest that addition of 4DF increases speed in clot formation and clot strength in diluted as well as in undiluted blood with capability of enabling almost normal coagulation even under conditions of 50% HAES dilution. The main mechanism of 4DF due to its specific molecular structure is capability to absorb water from the bleeding site. This attribute results to the relatively large surface of 4DF micro particles. After application of 4DF cellular components of blood like thrombocytes and clotting factors are concentrated on the primary site of bleeding.
Speed of coagulation is directly dependent on concentration of coagulation factors and the potential of thrombin generation. In this content thrombin generation and formation plays an important role in initiating first clot formation28. Thrombin activates platelets and converts fibrinogen to active fibrin[29]. The elevated concentration of coagulation factors and thrombocytes on the site of bleeding after water retraction by 4DF accelerated speed of coagulation. According to speed of this reaction CT is affected in its length in seconds. A dose depending reduction of the CT respectively an increase in coagulation speed represents the mechanism described prior.
4DF increases clot strength. Most important components for clot strength respectively firmness are platelets with normal function, FXIII and fibrinogen30. An increased concentration of these main determinants for cloth strength is proportional to an increase in clot firmness. We found a concentration effect of 4DF which also seems important for clot strength. 4DF has the ability to form a gel when absorbing liquid blood components. This gel seems able to stabilize the coagulum.
After adding 20 mg (normal dose) 4DF ROTEM was not able to assess regular clot formation resulting into irregular curves (example in fig.1). We interpret this phenomenon as highly indicative for massive decreased coagulation time and increased clot firmness. However, these irregular curves and consecutive impossibility of ROTEM in assessment of such instant massive coagulation led to retraction of 20mg values from statistical analysis. However, we suspect further relevant improvement of clot formation speed and clot strength beyond dosages of 10mg 4DF since further application of 4DF could lead to even more increased fluid retraction resulting in higher local concentration of coagulation factors and following further increase of coagulation speed. Moreover, in a clinical setting use of the normal dose (20mg) might be unproblematic due to the reported high biocompatibility of 4DF[13].
Dilution of coagulation factors is known as one possible cause of clinical coagulopathy in trauma[32]. Beside generally reduction it is actually not well understood how simple reduction of all single factors effects procoagulant, or anticoagulant status. Monroe calculated a model with a 37% reduction in single factor concentration to result in a 75% reduction in overall complex activity[33].
Dilution with colloid infusion and in particular hydroxyethyl starch is not only known for a reduction of coagulation factors. Hydroxyethyl starch has a specific effect of impairment on the coagulation system: it impairs thrombin generation, platelet activation and fibrin formation[32]. Most relevant effect is impairment of fibrin polymerization. Furthermore, HAES has significant effects in impairment of platelet function. This effect represents an extracellular coating of the platelet surface with colloidal macromolecules. This coating leads to an inhibition in conformational changes and interaction of glycoproteins with their ligands, such as fibrinogen[34,35].
Thus, 50% HAES dilution was used as a model for impaired coagulation to assess 4DF effects even under these clinically relevant conditions. As described capability of 4DF in absorbing liquids in wounds plays a major role. Increasing concentration of coagulation factors and platelets accelerate clot strength and speed of coagulation even under dilution. Since 4DF forms a gel by absorbing liquid blood components and consecutively increases local concentration of coagulation factors we suspect this increase in concentration to directly increase speed of clot formation and clot stabilization. Furthermore, the gel itself can build a mechanical barrier to the wound site which could lead to further reduction of blood loss.
Beside TIC further indications could be of interest as for example the use of 4DF in Jehovah’s Witnesses or other patients that do not accept blood-, plasma- or platelet transfusion. In this situation liquid substitution by crystalloids or colloids can lead to dilution and coagulopathy is a common resulting clinical finding. 4DF could be an option for this situations[36].
Another possible indication could be in patients with chronic liver disease that present a decrease of both procoagulant and anticoagulant factors and a high risk of bleeding and thrombotic events[37]. 4DF could decrease the risk of bleeding without having any intravascular effects. A possible hypercoagulative effect of any intravenously applicated procoagulant agent could be avoided[38].
The present study is an in vitro study and thus has limitations. We used a ROTEM-based model to measure influence of 4DF on coagulation. Since tissue factor is the primary physiologic initiator of coagulation the EXTEM test appears most appropriate to measure coagulation alterations comparable to in vivo coagulation as it is activated by recombinant tissue factor. However, in vivo factors like acidosis, hypothermia and in particularly hypoperfusion with endothelia mediated activation of the protein C pathway and resulting hyperfibrinolysis is missing in this model2. In ROTEM, blood samples have no interaction of the endothelium with coagulation factors. Furthermore, there is no change in pressure or bloodstream as in active bleeding.
Furthermore, dilution of blood with 50% HAES is not exactly comparable to a blood sample of patients with a traumatic induced coagulopathy (TIC) and dilutional coagulopathy. All this factors are limitations of this study and might reduce explanatory power of the data. We suggest further research to prove in vivo efficacy.
5. Conclusion
Application of 4DF significantly increases speed of coagulation and clot firmness. We demonstrated a dose depended improvement of coagulation - even under conditions of dilutional coagulopathy.
With the use of normal dosed 4DF clot firmness in 50% HAES diluted blood is comparable to that of a native coagulum and might be capable to support other surgical measures in acute bleeding patients.
We speculate that higher doses of 4DF in HAES dilution are needed to support sufficient coagulation. Further clinical trials are necessary to prove in vivo efficacy.
References
- Cap, A. & Hunt, B. J. The pathogenesis of traumatic coagulopathy. Anaesthesia 70, 96– e34 (2014).
- Maegele, M., Schöchl, H. & Cohen, M. J. An Update on the Coagulopathy of Trauma. Shock 41, 21–25 (2014).
- Teixeira, P. G. R. et al. Preventable or potentially preventable mortality at a mature trauma center. The Journal of Trauma: Injury, Infection, and Critical Care 63, 1338–1337 (2007).
- Kleber, C., Giesecke, M. T., Tsokos, M., Haas, N. P. & Buschmann, C. T. Trauma-related Preventable Deaths in Berlin 2010: Need to Change Prehospital Management Strategies and Trauma Management Education. World J Surg 37, 1154–1161 (2013).
- Brohi, K., Singh, J., Heron, M. & Coats, T. Acute Traumatic Coagulopathy. The Journal of Trauma: Injury, Infection, and Critical Care 54, 1127–1130 (2003).
- Maegele, M. et al. Early coagulopathy in multiple injury: An analysis from the German Trauma Registry on 8724 patients. Injury 38, 298–304 (2007).
- Rossaint, R. et al. The European guideline on management of major bleeding and coagulopathy following trauma: fourth edition. Critical care (London, England) 20, 100–100 (2015).
- Sachs, M. Die Methoden der Blutstillung in ihrer historischen Entwicklung. hämostaseologie 20, 83– 89 (2000).
- Achneck, H. E. et al. A Comprehensive Review of Topical Hemostatic Agents. Annals of Surgery 251, 217–228 (2010).
- Khoshmohabat, H., Paydar, S. & Kazemi, H. M. Overview of Agents Used for Emergency Hemostasis. Trauma (2016).doi:10.5812/ traumamon.26023
- Grissom, T. E. & Fang, R. Topical hemostatic agents and dressings in the prehospital setting. Current Opinion in Anaesthesiology 28, 210– 216 (2015).
- Tschan, C. A., Niess, M., Schwandt, E. & Oertel, J. Safety and Efficacy of Microporous Polysaccharide Hemospheres in Neurosurgery. Operative Neurosurgery. 2011; 69, ons49– ons63.
- Poehnert, D. et al. Evaluation of the biological tolerability of the starch-based medical device 4DryField(R) PH in vitro and in vivo a rat model. Journal of Biomaterials Applications. 2015; 30, 463–471.
- Poehnert, D. et al. Evaluation of the Effectiveness of Peritoneal Adhesion Prevention Devices in a Rat Model. Int. J. Med. Sci. 2016; 13, 524–532.
- Korell, M., Ziegler, N. & De Wilde, R. L. Use of Modified Polysaccharide 4DryField ®PH for Adhesion Prevention and Hemostasis in GynecologicalSurgery:ATwo-Center Observational Study by Second-Look Laparoscopy. BioMed Research International 2016; 1–9.
- Korell, M. Combined Hemostasis and Adhesion Prevention with the Novel Agent 4DryField<sup>®</sup> PH—Initial Observations. 2014; SS 05, 533– 539.
- Karsch, J.-J., Berthold, M. & Breul, J. Evaluation of Lymphorrhea and Incidence of Lymphoceles: 4DryField® PH in Radical Retropubic Prostatectomy. Advances in Urology 2016; 1–7.
- Bickell, W. H. et al. Immediate versus delayed fluid resuscitation for hypotensive patients with penetrating torso injuries. N Engl J Med. 1994; 331, 1105–1109.
- Brohi, K., Singh, J., Heron, M. & Coats, T. Acute traumatic coagulopathy. J Trauma. 2003; 54, 1127–1130.
- Geeraedts, L. M. G., Jr, Pothof, L. A. H., Caldwell, E., de Lange-de Klerk, E. S. M. & D’Amours, S. K. Prehospital fluid resuscitation in hypotensive trauma patients: Do we need a tailored approach? Injury.2015; 46, 4–9.
- Driessen, A. et al. Prehospital volume resuscitation - Did evidence defeat the crystalloid dogma? An analysis of the TraumaRegister DGU® 2002– 2012. Scand J Trauma Resusc Emerg Med.2016; 1–8. doi:10.1186/s13049-016-0233-4
- Hußmann, B., Lefering, R. & Taeger, G. Influence of prehospital fluid resuscitation on patients with multiple injuries in hemorrhagic shock in patients from the DGU trauma registry. J Emerg Trauma Shock. 2011.
- Hussmann, B. et al. Prehospital Volume Therapy as an Independent Risk Factor after Trauma. BioMed Research International. 2015; 1–9.
- Hartert, H. Blutgerinnungsstudien mit der Thrombelastographie, einem neuen Untersuchungsverfahren. Klin Wochenschr. 1948; 26, 577–583.
- Ganter, M. T. & Hofer, C. K. Coagulation Monitoring: Current Techniques and Clinical Use of Viscoelastic Point-of-Care Coagulation Devices. Anesth. Analg. 2008; 106, 1366–1375.
- Perry, D. J., Fitzmaurice, D. A., Kitchen, S., Mackie, I. J. & Mallett, S. Point-of-care testing in haemostasis. British Journal of Haematology. 2010; 150, 501–514.
- Nielsen, V. G., Geary, B. T. & Baird, M. S. Evaluation of the contribution of platelets to clot strength by thromboelastography in rabbits: the role of tissue factor and cytochalasin D. Anesth. Analg. 2000; 91, 35–39.
- Mann, K. G., Butenas, S. & Brummel, K. The dynamics of thrombin formation. Arteriosclerosis. 2003. doi:10.1161/01. ATV. 0000046238.23903.FC
- Hoffman, M. & Monroe, D. M. A cell-based model of hemostasis. Thromb Haemost.2001; 85, 958–965.
- Lang, T. & Depka, Von, M. Diagnostische Möglichkeiten und Grenzen der Throm belastometrie/-graphie. hämostaseologie. 2006. doi:10.5482/ha-1132
- Levy, J. H., Szlam, F., Tanaka, K. A. & Sniecienski, R. M. Fibrinogen and Hemostasis. Anesth. Analg. 2012; 114, 261–274.
- Brummel-Ziedins, K., Whelihan, M. F., Ziedins, E. G. & Mann, K. G. The resuscitative fluid you choose may potentiate bleeding. The Journal of Trauma: Injury, Infection, and Critical Care. 2006; 61, 1350–1358.
- Monroe, D. M. Modeling the action of factor VIIa in dilutional coagulopathy. Thrombosis Research. 2008; 122, S7–S10.
- Hanke, A. A. et al. In Vitro impairment of whole blood coagulation and platelet function by hypertonic saline hydroxyethyl starch. Scand J Trauma Resusc Emerg Med. 2011; 19, 12.
- Kozek-Langenecker, S. A. Fluids and coagulation. Current Opinion in Critical Care. 2015; 21, 285–291.
- Lawson, T. & Ralph, C. Perioperative Jehovah's Witnesses: a review. British Journal of Anaesthesia.2015; 115, 676–687.
- Tripodi, A. & Mannucci, P. M. The coagulopathy of chronic liver disease. N Engl J Med. 2011; 365, 147–156.
- Tripodi, A. et al. An imbalance of pro- vs anti-coagulation factors in plasma from patients with cirrhosis. Gastroenterology. 2009; 137, 2105–2111.