Information
Journal Policies
Effect of Plasma-Activated Bleaching on Enamel Microhardness and Morphology
Esra Uzer Celik1*, Fatma Yilmaz2, Utku Kursat Ercan3, Fatma Ibis4
2.Department of Restorative Dentistry, Faculty of Dentistry, Pamukkale University, Kınıklı Campus, 20160, Pamukkale, Denizli, Turkey
3.Department of Biomedical Engineering, Faculty of Engineering and Architecture, Izmir Katip Celebi University, Cigli Main Campus, 35620 Cigli, Izmir, Turkey
4.Department of Biomedical Engineering, Faculty of Mechanical, Maritime and Materials Engineering, Delft University of Technology, Mekelweg, 2628, CD Delft, Nederlands
Copyright : © 2018 Authors. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Objectives: This in vitro study aimed to evaluate the effect of nonthermal atmospheric pressure plasma (NAPP)-activated hydrogen peroxide (HP) and deionized water (DW) on enamel microhardness and enamel surface morphology.
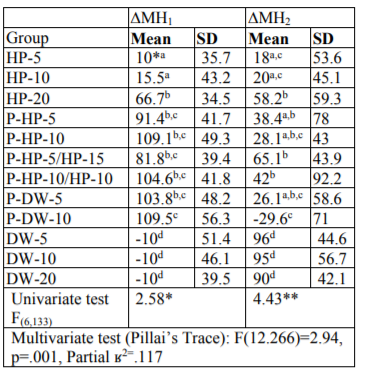
Methods: Two hundred-forty human enamel-dentin samples were randomly divided into 12 groups (n = 20): (1) HP for 5 minute (HP-5), (2) HP-10, (3) HP-20, (4) Plasma-HP-5, (5) P-HP-10, (6) P-HP-5/HP-15, (7) P-HP-10/HP-10, (8) P-DW-5, (9) P-DW-10, (10) DW-5, (11) DW-10, and (12) DW-20. Using a microhardness tester with a Vickers penetrator, enamel indentations were made before bleaching and 1 and 14 d after bleaching. ΔMH1 and ΔMH2 indicated the hardness changes after 1 and 14 d, respectively. The surface morphology of 72 human enamel-dentin samples (n=3) was assessed using a scanning electron microscope (SEM) (magnifications of 500×, 1500×, 2500× and 5000×). The ΔMH1 and ΔMH2 values were analyzed by a multiple analysis of variance (MANOVA) and Tukey’s HSD post-hoc tests (p< 0.05).
Results: The ∆MH1 and ΔMH2 values of the P-DW-10 group were significantly different from that of the other groups (p< 0.05). The surface morphology of the plasma-treated groups was smoother than that of the untreated groups.
Conclusion: NAPP-activated HP and DW have limited and reversible effects on dental enamel.
Keywords: Nonthermal atmospheric pressure plasma, vital bleaching, microhardness, surface morphology,Dental Science
1. Introduction
Although a light source is widely used in dental bleaching to increase the whitening process, its use is controversial, with some researchers not finding any benefits of light-source devices[1]. Light sources raise the temperature of the tooth, potentially causing unwanted morphological and structural changes on the enamel surface[1].
In recent years, nonthermal atmospheric pressure plasma (NAPP) has been used as an activator rather than a light source, as NAPP does not produce excessive heat during the bleaching process [2]. Plasma is the fourth state of matter and a highly reactive material because it is composed of charged particles and radicals and has a strong electric field. Lee et al. [3] used a NAPP jet to increase the bleaching efficacy of hydrogen peroxide (HP). As shown in another study, the energetic ions, free electrons, and hydroxyl radicals produced by the plasma jet increased the bleaching effectiveness of HP and carbamide peroxide (CP) [2]. However, the jet system was found to be impractical because the gas flow resulted in the removal of gel from the enamel surface.
HP is toxic to soft tissues [4]. It has been widely reported that the antimicrobial activity of water is increased when treated with plasma due to the diffusion of plasma-generated species, which may also play role in tooth bleaching [5]. Therefore, in the present study, deionized water (DW) was used as a transfer (or diffusion) medium of plasma-generated species for tooth bleaching. The bleaching process can induce changes in the mineral content and micromorphology of tooth enamel, including decreased enamel microhardness due to the loss of calcium and phosphate [6]. Using in vitro studies, the effects of the bleaching process can be evaluated by measuring microhardness values after bleaching applications [7]. To the best of our knowledge, there are no data on the effect of NAPP-activated HP and DW on the microhardness and surface morphology of enamel.
Therefore, in the present study, we investigated the effect of NAPP-activated HP gel (40%) and DW on enamel microhardness and enamel surface morphology. Nonthermal, atmospheric pressure dielectric barrier discharge (DBD) air plasma was used instead of a plasma jet to eliminate the removal of bleaching agents from the enamel surface. The null hypothesis of this study was that there would be no difference between the enamel microhardness and surface morphology of the teeth treated with the plasma-activated and nonactivated agents.
2. Materials And Methods
The study protocol was approved by the Ethical Committee of the Faculty of Medicine, XXX University (#2015-215). In this study, microhardness tests and scanning electron microscope (SEM) evaluations were performed. The experiments were performed in the Research Laboratory of the Faculty of Dentistry and Medical Plasma Laboratory of the Faculty of Engineering and Architecture at XXX University and in the Characterization Laboratory of the Faculty of Metallurgy and Materials Engineering at XXX University.
One hundred-twenty extracted human molars were used. Soft tissue remnants and calculi on the teeth were removed using a cretuar, and the teeth were then stored in 10% formalin for 7 d [8]. The crowns were cut with a slow-speed diamond-cutting saw (Isomet Diamond Wafering Blades, Buehler, IL, USA) in the mesio-distal direction under water cooling. Two hundred-forty samples were obtained. These were embedded in cold self-curing acrylic, so that the enamel and dentin were exposed to the external environment to maintain an electrical field. Enamel slabs were flattened in a polishing machine (Mecatech 234, Presi, Grenoble, France), with decreasing granulations (240, 400, 600, and 1200) of water-abrasive paper under water cooling and polished with diamond pastes of sequentially decreasing granulation (6, 3, 1 and 1/2 μm) on felt discs.
The samples were divided into the following 12 groups, with 20 samples in each group according to the bleaching methods and application periods applied:
Group 1 (HP-5): A 0.5–1.0 mm thick layer of 40% HP (Opalescence® Boost PF 40%, Ultradent Inc., UT, USA) gel was applied to the labial surfaces of the samples for 5 min, followed by incubation of the HP gel-covered samples in an incubator at 37° C for 5 min.
Group 2 (HP-10): The same procedures as those used in group 1 were applied but for 10 min.
Group 3 (HP-20): The same procedures as those used in group 1 were applied but for 20 min.
Group 4 (P-HP-5): 40% HP was applied to the samples, and they were then treated for 5 min with plasma.
Group 5 (P-HP-10): In this group, the same procedures were used as those in group 4 but for 10 min.
Group 6 (P-HP-5/HP-15): After 40% HP was applied to the samples, the gel was activated by plasma as described above for 5 min. Following the 5 min-activation by plasma, the HP gel-covered samples were placed in an incubator at 37° C for 15 min.
Group 7 (P-HP-10/HP-10): The plasma treatment was applied as described above for 10 min. Following the 10 min-activation by plasma, the HP gel-covered samples were placed in an incubator at 37° C for 10 min.
Group 8 (P-DW-5): DW was applied to the samples and then activated by plasma as described above for 5 min. To produce an electric field, the plasma device was switched off every 1 min, and then 1 drop of DW was placed on the buccal surfaces of the samples.
Group 9 (P-DW-10): In this group, the same procedures as used in group 8 were applied but for 10 min.
Group 10 (control group) (DW-5): The samples were placed in a DW-filled container and then kept in an incubator for 5 min at 37° C as a control group for the HP-5, P-HP-5 and P-DW-5 groups.
Group 11 (control group) (DW-10): The samples were placed in a DW-filled container and then moved to an incubator for 10 min at 37° C as a control group for the HP-10, P-HP-10, and P-DW-10 groups.
Group 12 (control group) (DW-20): The samples were placed in a DW-filled container and then moved to an incubator for 20 min at 37° C as a control group for the HP-20, P-HP-5/HP-15, and P-HP-10/HP-10 groups.
After each application, the samples were cleaned with a brush under water for 30 s. The samples were polished using polishing discs (Sof-LexTM Polishing Discs, 3M ESPE, MN, USA) under water cooling. The fluoride gel (Elmex Gel, Gaba, Lörrach, Germany) was then applied to the samples for 4 min. The samples were immersed in artificial saliva and kept in an incubator at 37° C until the microhardness measurements. The saliva was refreshed every day.
In the present study, all the plasma treatments were performed using an alternating current (AC) microsecond-pulsed power supply. Nonthermal, atmospheric pressure DBD air plasma was generated at a frequency of 1.5 kHz, peak-to-peak voltage of 22 kV, and pulse duration of 5 µs, maintaining a 2-mm discharge gap.
The Vickers hardness test was used to measure hardness changes that occurred in the tooth enamel after bleaching. Enamel indentations were made before bleaching and 1 and 14 d after bleaching using a Vickers penetrator and a 10-g load for 30 s, with the indentations made 500 µm apart from each other. In each period, 3 indentations were made, and the mean was used in the statistical analysis [9]. The microhardness was tested using an HMV-2000 device (Shimadzu, MD, USA). The microhardness was assessed using the following formulas: ΔMH1 = MH1 - MH2 and ΔMH2 = MH3-MH1, where MH1 was the microhardness value before bleaching, MH2 was the microhardness value 1 d after bleaching, and MH3 was the microhardness value 14 d after bleaching [10].
The surface morphology of 36 extracted human molars (3 from each of the 12 groups) was evaluated by scanning electron microscopy. The buccal and lingual/palatal samples of each tooth were matched. The samples were embedded in cold self-curing acrylic. One-half of each sample was bleached, and no bleaching procedure was applied to the other half of the sample. After the bleaching procedures, to dehydrate the samples, they were kept in a vacuum desiccator (VDC-31, Jeiotech, Seoul, Korea) for 24 h. The tooth samples were coated with gold-palladium under high vacuum using a sputter coater (SC7620 Mini Sputter Coater and Glow Discharge System, Quorum Technologies Ltd., Laughton, Lewes, East Sussex, UK) to make them conductive prior to the SEM examination. Following these procedures, the samples were analyzed using an SEM (JSM-5400, Jeol, Tokyo, Japan) (magnifications of 500×, 1500×, 2500× and 5000×).
The Shapiro–Wilk test was performed to examine whether the data were normally distributed. The results of the ΔMH1 and ΔMH2 microhardness measurements were analyzed using a multiple analysis of variance (MANOVA) and Tukey’s HSD post-hoc tests. The results were evaluated at the 95% confidence interval and p < 0.05 level.
3. Results And Discussion
The mean values of the ΔMH1 and ∆MH2 data in each group are given in Table 1.
The reduction in microhardness after bleaching (ΔMH1) was significantly higher in all the experimental groups compared to that of the control groups (p < 0.05). There was no statistically significant difference in the ΔMH1 values among the control groups, whereas the difference in ΔMH1 values among the experimental groups was statistically significant (p < 0.05) (Table 1). Among the experimental groups, HP-5 and HP-10 showed the lowest reduction in microhardness after bleaching (p < 0.05). There was no statistically significant difference in the microhardness of the HP-20 and plasma-activated groups, except in the P-DW-10 group. The ΔMH1 value of the P-DW-10 group was significantly higher than that of the HP-5, HP-10, and HP-20 groups (p < 0.05), whereas there was no statistically significant difference in the ΔMH1 values of the P-DW-10 group and those of the other plasma-activated groups.
The return of the microhardness values of the study groups to baseline values after remineralization (ΔMH2) was statistically different (p < 0.05) (Table 1). In all the experimental groups, the ΔMH2 values were significantly lower compared to those of the control groups (p < 0.05). The microhardness values of all the experimental groups, except those in the P-DW-10 group, returned to baseline values after remineralization. The ΔMH2 value of the P-DW-10 group was significantly lower than that of the HP-5, HP-10, and HP-20 groups (p < 0.05). In the plasma-activated groups, the ΔMH2 values of the P-DW-10 group were lower than those of the P-HP-5, P-HP-5/HP-15, and P-HP-10/HP-10 groups (p < 0.05).
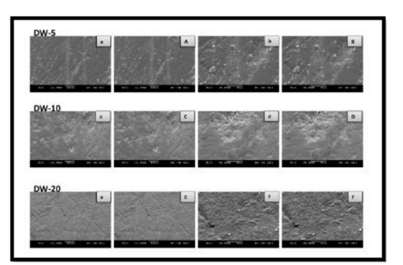
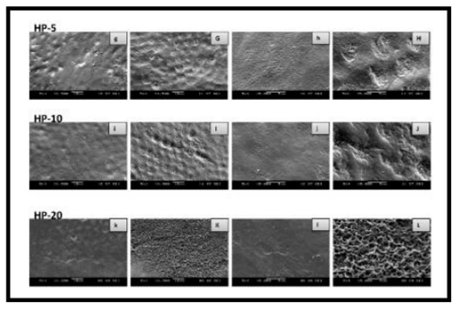
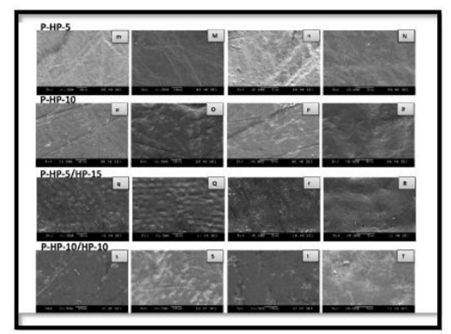
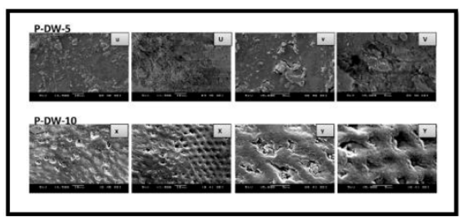
As shown by the SEM images, some alterations were observed on the enamel surface after bleaching in the experimental groups, whereas no differences were observed among the control groups (Figures 1–4). The SEM images of the HP-5 and HP-10 groups revealed partial loss of the enamel surface (Figure 2). The SEM images of the HP-20 group revealed clear porosity on the enamel surface and enamel prisms, with an atypical crystal appearance. The resulting surface roughness of the HP-20 group was similar to that of the images of etched enamel (Figure 2). The HP-5 group showed less porosity than the HP-10 group, and the HP-10 group showed less porosity than the HP-20 group. The surface morphology of the samples in the plasma-activated HP groups was relatively smooth (Figure 3). Although the plasma-activated DW groups (P-DW-5 and P-DW-10) exhibited less porosity than the nonactivated HP groups (HP-5, HP-10, and HP-20), their surface morphology was clearly rougher than that of the plasma-activated HP groups (P-HP-5, P-HP-10, P-HP-5/HP-15, and P-HP-10/HP-10) (Figures 2–4).
Based on the findings of the present study, the null hypothesis that there would be no difference between the enamel microhardness and surface morphology of the plasma-activated and nonactivated samples was rejected. It is generally accepted that dental bleaching cause a transient decrease in the abrasion resistance and microhardness of enamel immediately after the procedure [11], although some authors have presented discordant reports on this subject [12]. Similarly, in the present study, the microhardness values decreased significantly in the nonactivated HP groups 1 d after bleaching. As shown by in vitro studies, the demineralization effect of HP was directly proportional to the contact time [13]. In the present study, although there was no difference between the ΔMH1 values of the HP-5 and HP-10 groups, the ΔMH1 values of these groups were lower than those of the HP-20 group.
Araujo et al. [14] evaluated the effect of 35% HP activated by different light sources (LED, a halogen light, and an argon laser) on enamel microhardness and found no negative effect of the light source on microhardness. Mielczarek et al.[15] reported that the amount of insoluble inorganic structures depended on the contact time of the bleaching agents with the tooth surface. In the present study, there was no difference in the ΔMH1 values of the HP-20 group versus those of the plasma-activated groups, except the P-DW-10 group. Although the contact time of the gel was reduced in the P-HP-5 and P-HP-10 compared to that of the HP-20 groups, there was no difference in the microhardness of these groups. Based on these findings, it can be concluded that the additional bleaching activity, high oxidative capacity, and formation of nitric acid and nitrous acid by 5 min and 10 min plasma of HP can cause microhardness decreases similar to those seen in the HP-20 group [16]. In the P-DW-10 group, the demineralization after bleaching was higher than that in the HP-20 group. The high demineralization effect in the P-DW-10 group could be attributed to the high oxidative capacity of plasma. During plasma treatment of DW, ozone, reactive oxygen species, charged particles, and strongly oxidizing radicals are generated and diffused in water [5,17]. In addition, plasma-activated DW has an acidic pH. However, in the present study, the ∆MH1 value of the P-DW-5 group was similar to that of the other experimental groups, except P-DW-10. The higher demineralization effect may be associated with the longer plasma application.
Demineralization that occurs after bleaching can be repaired by absorption and deposition of salivary components [18]. In the present study, the microhardness values of all the experimental groups, except the P-DW-10 group, returned to baseline values 14 d after bleaching, pointing to remineralization of the samples. The samples in the P-DW-10 group were not thoroughly demineralized after storage for 14 d in artificial saliva, and the lowest remineralization was observed in this group. Basting et al.[19] reported that the remineralization ability of saliva was time dependent. When enamel is bleached with P-DW-10, remineralization of the surface may take longer than when enamel is bleached with a conventional gel technique, without light activation.
In the present study, the SEM analysis of the surface morphology after bleaching revealed some alterations, depending on the method used. Pinto et al. [11] reported that the surface roughness increased after bleaching with different bleaching agents. Another SEM study clearly observed erosion and porosity on the enamel surface after bleaching [20]. In addition, the calcium/phosphate ratio was decreased, and superficial alterations were observed on bleached enamel [21]. The present SEM analysis revealed no alterations on the enamel surfaces of the control groups, but enamel porosity was clearly observed in the HP-20 group, as reported in the literature [22].
In an in vitro study, the appearance of bleached enamel surfaces was shown to be similar to that of etched enamel surfaces, depending on the type, concentration, and application process of the bleaching agent [20]. In the present study, less porosity was observed in the HP-5 and HP-10 groups than in the HP-20 group. This finding was consistent with the microhardness results and indicated that the demineralization caused by the 40% HP agent was directly proportional to the application time. Most likely, these changes are reversible in the presence of human saliva and may not have clinical significance [23].
As compared to the nonactivated HP groups, the plasma-activated HP group showed no demineralized areas and had a relatively smooth surface morphology. Nam et al.[16] also reported smoother surfaces in samples bleached with plasma-activated 15% CP as compared to nonactivated 15% CP and attributed this to the elimination of most precipitated proteins, such as the smear layer and discoloring agents, on the tooth surface. These findings might be related to the bleaching ability of plasma. These results indicate that the application of plasma does not cause morphological defects on the enamel surface and effectively removes discoloring agents deposited on the tooth surface.
In the plasma-activated DW groups, some enamel particles were removed from the surface, and “half-floating” particles appeared. These observations could be explained by the formation of ozone, reactive oxygen species, charged particles, and strongly oxidizing radicals in the plasma-activated DW [5,24].
Smoother surfaces were observed in the plasma-activated HP groups compared to the plasma-activated DW groups, which may possibly be the result of the pH neutralizing effect of the bleaching gel. Moreover, the presence of remineralizing agents, such as sodium fluoride and potassium nitrate, in the HP bleaching agent may have quenched the acid products generated during the plasma treatment.
4. Conclusions
Other than in the P-DW-10 group, the demineralization that occurred in the plasma-activated groups was similar to that observed in the HP-20 group. HP-20 is a commonly used conventional in-office bleaching technique. The microhardness values of the P-DW-10 group did not return to baseline values after the remineralization period. Among all the experimental groups, the greatest surface roughness was observed in the HP-20 group, and relatively smooth surface morphology was observed in all the plasma-activated HP and DW groups. NAPP-activated HP and DW have limited and reversible effects on hard tissues and may be safely used for dental bleaching.
Acknowledgements
This work was funded by the Scientific Research Projects Council at Izmir Katip Celebi University (grant number 2016-ÖDL-SABE-0005).
References
- Hein D. K., Ploeger B. J., Hartup J. K., Wagstaff R. S., Palmer T. M. and Hansen L.D., In-office vital tooth bleaching-what do lights add?, Compend. Contin. Educ. Dent. 24, 340-352 (2003).
- Iza F., Kim G. J., Lee S. M., Lee J. K., Walsh J. L., Zhang Y. T. and Kong M. G., Microplasmas: sources, particle kinetics, and biomedical applications, Plasma Process. Polym. 5, 322-344 (2008).
- Claiborne D., McCombs G., Lemaster M., Akman M. A. and Laroussi M., Low‐ temperature atmospheric pressure plasma enhanced tooth whitening: the next‐generation technology, Int. J. Dent. Hyg. 12, 108-114 (2014).
- Lee H. W., Kim G. J., Kim J. M., Park J. K., Lee J. K. and Kim G. C., Tooth bleaching with nonthermal atmospheric pressure plasma, J. Endod. 35, 587-591 (2009).
- B. Halliwell and J. M. C.Gutteridge, Free radicals in biology and medicine. 5 th ed. New York, U. K: Oxford University Press Inc., 2015, ch 1, pp: 23-25.
- Burlica R., Kirkpatrick M. J. and Locke B. R., Formation of reactive species in gliding arc discharges with liquid water, J. Electrostat. 64, 35-43 (2006).
- Potočnik I., Kosec L. and Gašperšič D., Effect of 10% carbamide peroxide bleaching gel on enamel microhardness, microstructure, and mineral content, J. Endod. 26, 203-206 (2000).
- Feagin F., Koulourides T. and Pigman W., The characterizationofenamelsurface demineralization, remineralization, and associated hardness changes in human and bovine material, Arch. Oral Biol. 14, 1407-1417 (1969).
- Kumar M., Sequeira P. S., Peter S. and Bhat G. K., Sterilisation of extracted human teeth for educational use, Indian J. Med. Microbiol. 23, 256-258 (2005).
- De Abreu D. R., Sasaki R. T., Amaral F. L. B., Flório F. and Basting R. T., Effect of home‐use and in-office bleaching agents containing hydrogen peroxide associated with amorphous calcium phosphate on enamel microhardness and surface roughness, J. Esthet. Restor. Dent. 23, 158-168 (2011).
- Navimipour E. J., Kimyai S., Nikazar S. and Ghojazadeh M., In vitro evaluation of the effect of delaying toothbrushing with toothpaste on enamel microhardness subsequent to bleaching the teeth with 15% carbamide peroxide, Oper. Dent. 37, 87-92 (2012).
- Pinto C. F., Oliveira R. D., Cavalli V. and Giannini M., Peroxide bleaching agent effects on enamel surface microhardness, roughness and morphology, Braz. Oral Res. 18, 306-311 (2004).
- Seghi R. R. and Denry I., Effects of external bleaching on indentation and abrasion characteristics of human enamel in vitro, J. Dent. Res. 71, 1340-1344 (1992).
- Tezel H., Ertaş O. S., Ozata F., Dalgar H. and Korkut Z. O., Effect of bleaching agents on calcium loss from the enamel surface, Quintessence Int. 38, 339-347 (2007).
- Araujo F. O., Baratieri L. and Araújo E., In situ study of in-office bleaching procedures using light sources on human enamel microhardness, Oper. Dent. 35, 139-146 (2010).
- Mielczarek A., Klukowska M., Ganowicz M., Kwiatkowska A. and Kwaśny M., The effect of strip, tray and office peroxide bleaching systems on enamel surfaces in vitro, Dent. Mater. 24, 1495-1500 (2008).
- Nam S. H., Lee H. J., Hong J. W. and Kim G. C., Efficacy of nonthermal atmospheric pressure plasma for tooth bleaching, ScientificWorldJournal 2015, 5817 (2015).
- Brisset J. L., Moussa D., Doubla A., Hnatiuc E., Hnatiuc B., Kamgang Youbi G., Herry J. M., Naïtali M. and Bellon Fontaine M. N., Chemical reactivity of discharges and temporal post-discharges in plasma treatment of aqueous media: examples of gliding discharge treated solutions, Ind. Eng. Chem. Res. 47, 5761-5781 (2008).
- Amaechi B. T. and Higham S. M., In vitro remineralisation of eroded enamel lesions by saliva, J. Dent. 29, 371-376 (2001).
- Basting R. T., Rodrigues A. L. and Serra M. C., The effects of seven carbamide peroxide bleaching agents on enamel microhardness over time, J. Am. Dent. Assoc. 134, 1335-1342 (2003).
- Zalkind M., Arwaz J. R., Goldman A. and Rotstein I., Surface morphology changes in human enamel, dentin and cementum following bleaching: a scanning electron microscopy study, Endod. Dent. Traumatol. 12, 82-88 (1996).
- Rotstein I., Dankner E., Goldman A., Heling I., Stabholz A. and Zalkind M., Histochemical analysis of dental hard tissues following bleaching, J. Endod. 22, 23-25 (1996).
- McGuckin R. S., Babin J. F. and Meyer B. J., Alterations in human enamel surface morphology following vital bleaching, J. Prosthet. Dent. 68, 754-760 (1992).
- Sa Y., Wang Z., Ma X., Lei C., Liang S., Sun L., Jiang T. and Wang Y., Investigation of three home-applied bleaching agents on enamel structure and mechanical properties: an in situ study, J. Biomed. Opt. 17(3), 035002, (2012).
- Ikawa S., Kitano K. and Hamaguchi S., Effects of pH on bacterial inactivation in aqueous solutions due to low-temperature atmospheric pressure plasma application, Plasma Process. Polym. 7, 33-42 (2010).