Information
Journal Policies
Prevention of Anal Carcinoma in HIV/AIDS Patients
Atul Kakar*, Atul Gogia, Amit Ganwani
Copyright : © 2018 . This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
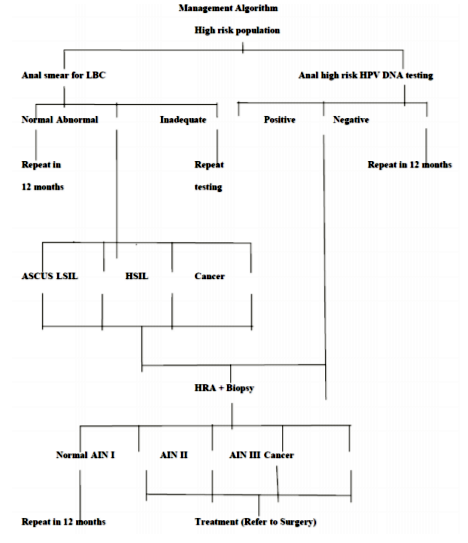
Life expectancy of HIV/AIDS patients has increased with the advent of antiretroviral therapy (ART) and prophylactic antibiotic therapy. Physicians are now focusing their attention from opportunistic infection to malignancies. The awareness of anal HPV infection, importance of its screening, vaccination, early diagnosis and treatment is lacking among the physicians who are treating People Living with HIV/AIDS (PLHA). Conventional Papanicolaou (Pap) smear has been replaced by Liquid Based Cytology (LBC) as it gives high cell yield and reduces compromising factors like air drying, fecal material and mechanical artifacts. Repeat testing can be done on the later date using same specimen. The specimen are collected from anal canal using cytobrush in LBC vial and HPV DNA testing is usually done by Hybrid Capture II technique based on antibody capture and chemiluminescent signal detection. Anal cytological abnormalities are reported as per the guidelines of ‘The Bethesda System for reporting anal cytology’. Patients with positive HPV DNA test and / or presence of anal cytological abnormalities should be further investigated by High Resolution Anoscopy (HRA) and anal biopsy to confirm the cytological changes. Aim of this article is to review the risk factors for anal cancer, its presentation, management options and anal cytological abnormalities. The authors suggest management algorithm for the same.
Anal; HIV; AIDS; HPV; Pap; LBC,AIDS
1. Introduction
There are two types of human immunodeficiency virus (HIV), HIV 1 and HIV 2. Persistent infection with this virus over a period of time leads to immune depletion and Acquired Immunodeficiency Syndrome (AIDS)[1]. It significantly increases the risk of opportunistic infections and malignancies that are rare in general population. Life expectancy of HIV/AIDS patients has increased with the advent of antiretroviral therapy (ART) and prophylactic antibiotic therapy. Our focus of attention has now shifted from opportunistic infection to malignancies. Lymphomas, Kaposi sarcoma, cervical and anal carcinoma are common malignancies in PLHA. In India, burden of the infection is significantly greater than the world average. Anal intraepithelial neoplasia (AIN) is a precancerous lesion that can progress to anal squamous cell carcinoma (ASCC). HIV infection is one of the major risk factor for development of anal carcinoma[2]. Most common variant is squamous cell type, whereas, adenocarcinoma and other skin cancer variants are uncommon. Over 90% of ASCC are attributable to infection with high risk strains of Human Papilloma Virus (HPV)[3]. Other risk factors for anal cancer are high risk behavior like men who have sex with men (MSM), multiple sexual partners, receptive anal intercourse, unprotected sex. Anal cancer and its precancerous lesions are underappreciated by most health care providers. Despite of low incidence, anal carcinoma has significant morbidity and mortality.
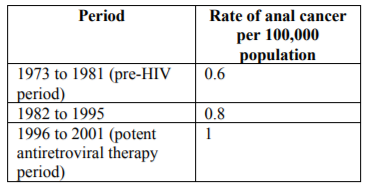
Prevalence of anal cancer in general population is difficult to estimate due to its low incidence and no recommended guidelines for screening. However, population at risk of anal carcinoma is identified and studied to determine its prevalence. Anal cancer accounts for 0.5% of all new cancer cases in United Stated. In 2018, in US, 8580 new cases of anal cancer are estimated. Based on 2011-2015 data number of new cases of anal cancer was 1.8 per 100,000 men and women per year. Incidence of anal cancer has increased and has almost doubled in the last 25 years in United States[4]. In a systematic review and meta-analysis incidence of anal cancer in HIV positive MSM was found to be 45.9 per 100,000 men, whereas in HIV negative MSM the incidence was found to be 5.1 per 100,000 men[5]. In the study on prevalence of anal squamous intraepithelial lesions in women it was found that abnormal anal cytological features were detected in 26% of HIV positive and 8% of HIV negative women[6]. In United Stated incidence rate of HPV related in situ anal cancer was found to be 6.3 per 100,000 person years and that of invasive anal cancer was found to be 12.3 per 100,000 person years[7]. Chiao et al studied the changing rate of anal cancer in general population from the period before the emergence of HIV infection to the development of potent antiretroviral therapy. They found that the rate of anal cancer has increased over the years. (Table1).
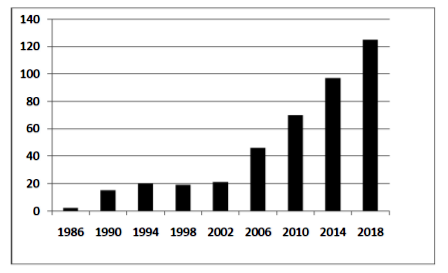
During same period, increasing trend in incidence of anal cancer in the HIV-infected MSM population was reflected by the decreasing females to males ratio from 1.6 to 1, to 1.2 to 1[8]. There has been significant increase in number of publications on anal cancer in HIV in recent years, highlighting the emerging burden of this malignancy in PLHA. (Figure 1).
Human Papilloma Virus is a double stranded DNA virus which commonly infects epithelial cells of skin and mucosa and leads to cellular proliferation. It is one of the most common sexually transmitted infections (STI) in HIV infected population. Over 100 types of HPV strains have been classified. Strains that affect anogenital region are divided into two groups, based on their oncogenic potential – high risk strains and low risk strains. The low-risk types of strains are non-oncogenic and can cause genital warts and benign cellular changes. Infections with high-risk types of strains most commonly HPV types 16 and 18, can cause low grade cellular changes, high-grade cellular changes, and squamous cell carcinoma[9]. Many cancers in the anogenital and oropharyngeal areas in both men and women are found to be caused by high risk strains of human papilloma virus (HPV) infection. Table-2 shows the percentage of various cancers assumed to be caused by HPV infection[10].
Most common site of HPV infection in cervical and anal canal is transition zone, it produces similar cytological abnormalities, hence The Bethesda System of reporting anal cytology is similar to cytology reporting in cervical cancer[11]. In a case-control study of anal cancer conducted by Daling et al 88% of all anal tumors were positive for HPV. Most frequent type detected in 73% of all tumors was HPV 16 followed by HPV 18 in 6.9% of all tumors. The incidence was even higher (97.7%) in men who were not exclusively heterosexual[12]. In 2013, Lin et al carried out a cross sectional study in Taiwan on men who have sex with men (MSM) to examine the prevalence of HPV infection and behavioral risk factors. It involved 279 men of which 166 (59.5%) were not infected with HIV and 113 (40.5%) were HIV infected. It was found that prevalence of high risk HPV infection was substantially higher in HIV infected men (adjusted odds ratio, 5.80; 95% confidence interval, 2.57-13.11), as compared to men with no HIV infection[13].
Human Immunodeficiency Virus preferentially infects cells of immune system leading to immune depletion and predisposes an individual to various opportunistic infection and malignancies. Prevalence of anal cancer is higher in HIV infected men because it is associated with increased incidence of HPV infection, inability to clear HPV infection leading to its persistence and simultaneous infection with multiple strains of HPV[14]. The incidence of anal carcinoma is 40 to 80 fold greater in HIV infected people as compared to general population.2 In a cross sectional study on prevalence of HPV infection in HIV positive men and women it was found that 81% of men had HPV infection with 64% high risk strains, in women HPV infection was present in 58% and high risk strains were present in 37%. In developed countries, prevalence of anal HPV infection and incidence of anal cancer is highest in HIV-positive men who have sex with men (MSM)[15,16]. A cross sectional study was carried out in Taiwan on men who have sex with men (MSM) to examine the prevalence of HPV infection and behavioral risk factors. It involved 279 men of which 166 (59.5%) were not infected with HIV and 113 (40.5%) were HIV infected. It was found that prevalence of high risk HPV infection was substantially higher in HIV infected men (adjusted odds ratio, 5.80; 95% confidence interval, 2.57-13.11), as compared to men with no HIV infection[17]. Anderson et al performed a study in Australia on prevalence of abnormal anal cytology and high risk HPV infection in HIV infected people with CD4 cell count >300 cells/micro l. They enrolled 126 HIV infected patients with median age of 45 years. Anal cytology swabs were collected in liquid based medium. They were used for assessment of cytological abnormalities and HPV typing by Hybrid Capture-2 assay. High risk HPV was found in 106 (84%) patients. Most common cytological abnormality found was ASCUS 32 (25%).They showed that high risk HPV types are commonly found in HIV positive population[18]. In a study on HIV positive women it was found that the risk of anal cytological abnormalities increased with decrease in CD4 cell count and increase in plasma HIV RNA viral load[6].
High risk behaviour includes unprotected sex, multiple sexual partners, men who have sex with men (MSM), receptive anal intercourse and intravenous drug abuse. This group of population is at higher risk of HPV and HIV transmission and HIV/HPV co-infection. This leads to increased incidence of anal cytological changes in this population. A cross sectional study was done in Korea on 201 HIV infected men irrespective of their sex behavior. The study enrolled 133 MSM and 68 MSW. Specimen was collected for cytology and HPV genotyping. Multivariable logistic regression was used to assess factors associated with anal HPV infection. Prevalence of HPV in their study was 82.7% in MSM and 51.5% in MSW. The most common high risk strain identified was HPV 16. MSM (47.4%) had higher prevalence of HR-HPV than MSW (25.0%; P = 0.002). MSM had abnormal anal cytology more commonly than MSW (42.9% vs.19.1%, P < 0.001). As per this study in HIV-infected MSM, the risk of any anal HPV infection was significantly increased with higher number of lifetime male sex partners, but age was a significant risk factor associated with anal HR-HPV infection[19]. Hernandez et al performed a multi centric study in 300 HIV positive MSM in India. The prevalence of anal HPV in MSM was estimated to be 95% (95% CI 91% - 97%). As per their study HPV 35 was the commonest strain detected (20%) followed by HPV 16 (13%). It was found that risk of anal HPV 16 infection was decreased in those taking antiretroviral medications (RR: 0.6(0.4 – 1.0)). Risk of any anal HPV infection was significantly lower in men who have increased number of vaginal sex partners. Receptive anal intercourse was found to be an important risk factor of infection with any anal HPV (RR: 1.2 (1.1 – 1.4)) and anal HPV 1616 (RR: 6.5 (1.8 – 10.7)). The study concluded that anal HPV infection was found in almost all Indian HIV positive MSM[20]. HIV-negative MSM have an estimated incidence rate of 35/100,000 [21] which is comparable to the incidence of cervical cancer in the general female population before widespread cervical Pap screening was introduced (40–50 cases/100,000)[22] and greater than the current incidence of cervical cancer (~9/ 100,000)[23]. Among HIV-positive MSM the incidence of anal cancer is thought to be even higher (~70–100 cases/100,000)[24].
Anal cancer is strongly associated with HPV infection, HIV infection and high risk behavior. However, other risk factors are identified which are also found to be associated with anal cancer. History of cervical intraepithelial neoplasia (CIN) or any gynecological cancer carries a high risk for anal intraepithelial neoplasia (AIN). Risk of AIN increases in presence of HPV associated dysplastic changes at other anatomical sites like cervix, vagina, vulva, penis, oropharynx, and larynx. This is due to chronic infection with high risk strains of HPV[25].In a study on HIV positive women it was found that risk of abnormal anal cytology increased in HIV positive women if they also had abnormal cervical cytology at the same visit[6].
Tobacco smoking is also identified as an important risk factor for anal cytological abnormalities. Gautier et al in a study found that regression of anal cytological abnormalities in presence of therapy failed to occur in smokers. Regression of lesions was seen in non smokers[26].
As in HIV infected individuals, those on chronic immunesuppresion due to chemotherapeutic agents, post transplant or any congenital immunodeficiency are also found to be at increased risk of anal cytological abnormalities[27]. This allows persistence of HPV infection which leads to anal cancer.
Majority of anal cancer are caused by persistent infection with HPV. Infection with low risk strains of HPV like type 6 and 11 lead to anal warts and papillomas. Persistent infection with high risk strains like type 16 and 18 lead to anal cytological abnormalities varying from ASCUS (Atypical Squamous Cells of Undetermined Significance) to invasive SCC (Squamous Cell Carcinoma). Patients with precancerous lesion or anal cancer may be asymptomatic or may present with a wide range of complaints, including the following: [28]
• Pain in anus or pelvis and anal bleeding (approximately half of patients)
• Sensation of rectal mass (approximately 30%)
• Local wetness and irritation
• Prolapse of tissue
• Incontinence of flatus or liquid or solid stool
• Obstipation
• Approximately 19% of patients wait 6 months or more to seek medical care after symptom onset. Once patients do present, delayed diagnosis or misdiagnosis is unfortunately frequent; in one review, 27% of anal cancers were diagnosed as haemorrhoids on the patient's first visit to a primary care provider[29].
Denial or reluctance on the part of many patients to seek medical attention for such complaints, as well as the similarity of those signs and symptoms to the manifestations of benign anorectal disease, help explain why anal cancer has historically had a very long lag time (up to 2 years on average) between initial symptoms and diagnosis[30]. More recent data suggest that the average time to diagnosis from onset of symptoms is 7.4 months and 3.2 months after the first visit to a physician. A patient complaint such as haemorrhoids, anal mass, or bleeding should always warrant a physical examination, with immediate referral to a colon and rectal surgeon for abnormal appearance, refractory disease, or presence of a mass.
Although the link between human papillomavirus (HPV) and AIN/anal cancer is well established, a lack of history of anoreceptive sexual practice does not rule out the possibility of HPV infection in the anal area, as there may be a significant "drip-down effect." In addition, non–HPV-related anal squamous cell carcinoma may also occur; such cases are usually attributed to chronic inflammation[31]. However, a history of anoreceptive sexual practice does increase the likelihood of HPV infection and its persistence in the anal area, [32] and also of AIN and anal cancer.
There are no recommended guidelines for screening for anal cancer in general population. However, screening for anal cancer has been recommended by experts in high risk population like those with HIV infection, women with cervical intraepithelial neoplasia, HPV related dysplasia at other sites and people with high risk behaviour. Most widely used test for screening is anal cytology and HPV DNA testing.
Anal cytology is now the standard of care according to many guidelines for screening in HIV/AIDS patient[33-35]. In 2001, Bethesda System Atlas first included anal cytology[11]. It has now gained acceptance as an important screening tool for anal cancer[36,37]. The Bethesda System (TBS) 2001 includes guidance on anal sample collection, its adequacy, use of Bethesda terminology for reporting of anal cytological abnormalities and their important morphologic characteristics. Majority of anal squamous cell carcinoma are attributable to persistent HPV infection.
It is now possible to detect and classify cytological changes in anal smear that precede anal squamous cell carcinoma. Single anal cytological specimen and a single cervical
cytology test have comparable sensitivity and specificity in detection of cytological abnormalities[38]. In a meta-analysis of anal cytology for HSIL, its sensitivity was found to be ranging from 69% to 93% and specificity ranging from 32% to 59% which was comparable to that of Pap tests[39].
Lindsey et al performed a study to validate anal Papanicolau (Pap) smears as a screening test for anal squamous cell carcinoma. They concluded that anal Pap smear is a valuable procedure that provides the opportunity for early detection of cytological abnormalities associated with HPV infection and guides appropriate follow up and intervention[40].
Anal sampling is done by cytobrush or dacron swab. Specimen should include the anal transformation zone and the non keratinized and keratinized squamous epithelium of the anal canal; hence sampling target extends from distal rectal vault proximally to the anal verge distally. Tip of cytobrush is moistened with water. The brush is inserted into the anal canal approximately 1.5 to 2 inches deep. Once inserted into the anus the brush is pulled out, rotating in spiral motion, applying some pressure to wall of anus, along the way. The cells, both rectal columnar and anal squamous cells collected on cytobrush are thoroughly rinsed and swirled in the LBC (Liquid Based Cytology) vial. The cytobrush is left in the LBC vial. Precancerous anal intraepithelial lesions are reported as per The Bethesda System of anal cytology reporting.
2. Cytological Interpretation
• Insufficient: Inadequate amount of cells present in the smear for the pathologist to make a determination. This can occur from the patient having had anal intercourse in recent hours, due to patient receiving an enema prior to sampling, or the cytobrush used for collection is not inserted far enough or rotated with enough pressure.
• NILM: Adequate amount of cells were sampled and these were normal in appearance. There is no evidence of HPV related changes.
• ASC-US: These cells show evidence of atypia. Cells are somewhat abnormal but the changes are not sufficient to classify as a squamous intraepithelial lesion.
• ASC-H: Another form of atypia. Cells are abnormal, but not enough to be classified as HSIL.
• LSIL: This report indicates mild dysplasia (also known as anal intraepithelial neoplasm, AIN).
• HSIL: Very abnormal cells that have dysplastic characteristics. Often described as moderate (AIN-2) or severe (AIN-3) dysplasia. This is considered precancerous. Invasive characteristics are not seen.
• SCC: This is invasive anal cancer.
• AGC: Changes are found in glandular cells that raise concern for the presence of precancer or cancer.
• Anal cancer is defined as cancer arising from the squamous epithelium of the anus.
Most commonly used test for detection for presence of high risk HPV DNA is Hybrid Capture II technique. This technique is based on antibody capture and chemiluminescent signal detection. Thirteen high risk strains that are most commonly tested for are 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68. The test is reported as positive or negative for high risk HPV infection. This test can be used individually or as a co-test with anal cytology for screening of anal cytological abnormalities.
Histology is the gold standard for diagnosis of anal cancer. If abnormal anal cytology is present and/or high risk HPV DNA test is positive, the next step in the diagnostic evaluation is HRA. This test allows direct visualization of the lesion and localizes the source of abnormal cells. It also helps obtain biopsy specimen for histological assessment which is essential to grade the severity of the disease. It involves use of anoscope for visualization of anal canal. A swab soaked in 3-5% acetic acid solution is inserted into the anal canal for few minutes. It causes acetowhite changes in areas of abnormal cells. Lugol’s iodine is then subsequently used for visualization of abnormal cells. But in this case abnormal cells are not stained while the normal cells stain with iodine. This is because iodine is glycophilic and dysplastic tissue lack glycogen. Any suspicious lesions, including condylomas, atypical surface configurations, punctuations, mosaicism, or atypical vessels, are then biopsied under direct visualization[42]. Areas with color changes seen on acetic acid staining that are subsequently found to be Lugol’s negative are highly suspicious for dysplasia, and are biopsied under direct visualization during HRA. The appearance of lesions under HRA with acetic acid staining is similar to those seen in cervical dysplasia[43]. HRA is considered superior to standard anoscopy as shown by Camus et al who reported that in a population of 102 patients [68% male; 57.3% HIV positive; mean 1.6 lesions (standard deviation 0.8) per patient] only 38.7% (65/168) of all lesions seen using HRA were visible with standard anoscopy[44]. In addition to detection of AIN, HRA also facilitates the application of therapies targeting AIN. HRA is ideally performed at centers specializing in its use as it requires expertise in identifying abnormal tissue appearance. The histology is reported as:[45]
• Normal – No evidence of dysplasia.
• AIN I – dysplastic cells occupying lower one third of epithelial thickness.
• AIN II – Dysplastic cells present in one third to two third thickness of anal epithelium.
• AIN III – Dysplastic cells present in complete thickness of anal epithelium with intact basement membrane.
• Invasive cancer - Dysplastic cells invading basement membrane.
Considering the high rates of dysplastic changes in HIV infected patients as compared to general population anal cytology screening is recommended at the initial evaluation and six months later for all patients with HIV infection. If these examinations are negative at both times point, the patient should be followed with yearly evaluations. If an initial or repeat cytology shows evidence of severe inflammation with reactive squamous changes, the next cytology testing should be performed at 3 months. If, at any time, cytology shows evidence of squamous intraepithelial lesions, high resolution anoscopy with biopsy should be performed[46]. Annual screening of HIV-infected MSM and screening every two to three years for HIV-uninfected MSM provided benefits in both life expectancy and cost effectiveness[47,48].
HPV types 16 and 18 cause nearly 90 percent of anal cancers; whereas, HPV types 6 and 11 cause approximately 90 percent of anogenital warts. Vaccines directed against the human papilloma virus (HPV) types associated with anal intraepithelial lesion and cancer in males have been developed. Three vaccines have been developed which target HPV. They difference is in the number of HPV types they contain. (Table 3)
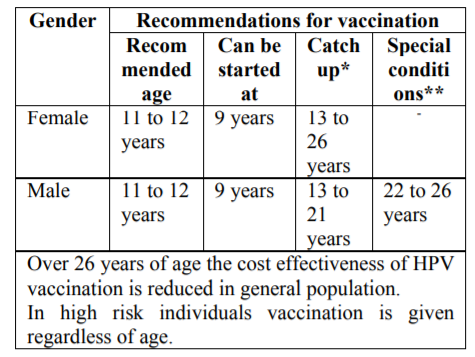
Recommendations for vaccination according to the Advisory Committee on Immunization Practices (ACIP) in the United States are as follows:[49-52]
*Catch up- Those who have not been previously vaccinated or who have not completed the vaccine series.
**Special condition- Those who are at high risk like MSM or immunocompromised state.
Schedule of vaccination: Three doses of vaccine are recommended intramuscularly at 0, 1 to 2 months and at 6 months[49-52].
Individuals may have prior history of acquiring HPV infection like abnormal cytology in cervix, anal canal, vulva or vagina or history of positive HPV test or genital warts. This infection could also be by strain not present in vaccine. HPV vaccination is still recommended in these individuals as it can provide protection against strains not acquired previously[49,50].
In a randomized trial in 4065 males, a quadrivalent vaccine was effective in preventing infection with HPV types 6, 11, 16, and 18 and prevented the development of external genital lesions[53]. In a study on 602 MSM on impact of HPV vaccine on development of AIN it was found that rates of persistent HPV infection were reduced by 95%.[54] More recently, a “nonavalent” vaccine has been developed, adding protection against HPV types 31, 33, 45, 52, and 58 to the previous four types, with rates of cervical and vulvar disease from these additional strains reduced from 1.6 per 1000 person-years in those receiving the quadrivalent vaccine to 0.1 per 1000 person-years in those receiving the nonavalent vaccine. These trials provide strong evidence that HPV vaccination is effective at preventing progression of AIN and cancer[55].
Use of barrier method of contraception provides protection from sexually transmitted infections. Avoiding multiple sexual partners, receptive anal intercourse, intravenous drug abuse, MSM and use of condoms not only decreases risk of HIV infection but also reduces transmission, persistence and simultaneous infection with multiple strains of HPV. All these being risk factors for anal cancer, practicing safe sex provides significant protection from HPV infection.
Treatment options available for anal cancer are as follows
Local application of bichloroacetic or trichloroacetic acid (TCA) is a reasonable option for small lesions (< 1 cm2 at the base). Topical application of TCA is generally well tolerated, but can occasionally be painful.
In a study of 72 HIV positive men with 98 HSIL, 79 percent of lesions resolved to normal epithelium or LSIL, and only two lesions (2 percent) required more than two treatments[56]. Similar results were obtained in a study of 54 men, 65 percent of whom were HIV positive[57]. In both studies, TCA was more effective in younger patients.
Preliminary data suggest that a course of topical fluorouracil may also be effective[58]. A 16-week course of self-administered twice weekly treatment resulted in a complete response in 18 and a partial response in eight (overall response rate 57 percent).
A course of self-applied, intra-anal imiquimod, an immune modulator, can result in pathological resolution of AIN in HIV-infected men who have sex with men (MSM) on HAART[59,60]. This approach may be best for patients with widespread, multifocal disease.
In one reported series, 64 men with HSIL were randomly assigned to imiquimod or placebo, applied to the anal canal three times per week for four months. Of the 28 patients given imiquimod, four had complete resolution and eight had the HSIL downgraded to LSIL. Only one of 25 patients on placebo had resolution of HSIL. At a median follow-up of three years, 61 percent of patients had sustained absence of HSIL.
For lesions that are too large for TCA, office-based infrared coagulation (IRC) can be used. This device is approved by the US Food and Drug Administration (FDA) for the treatment of haemorrhoids and for anal warts.
Treatment consists of the direct application of a 1.5 second pulse of irradiation in the infrared range to dysplastic anal epithelium, which results in tissue destruction to a depth of approximately 1.5 mm. The coagulated tissue can then be debrided using Tischler biopsy forceps. Possible procedure-related complications include immediate and delayed bleeding and infection[61].
IRC is not yet FDA approved for treatment of AIN. Multiple studies have demonstrated the safety and efficacy of IRC, in both HIV-infected and HIV-uninfected individuals[62-65]. As an example, in a retrospective study of 96 men, treatment with IRC was followed by recurrence in 62 percent of those who were HIV uninfected in a mean of 14 months, and 91 percent of those who were HIV infected[65]. Although multiple retreatments were required in the majority of cases, none of the men progressed to squamous cell carcinoma. There were no serious adverse events.
Hyfrecation is also commonly used to treat anal HSIL. It has a similar efficacy profile to IRC[66]. Some clinicians prefer it to IRC because it may be faster than IRC, particularly for large and keratotic lesions. It is also easier to use than IRC for perianal disease.
Argon plasma coagulation has been evaluated in a prospective pilot study of HIV-positive MSM. Sixty-five percent of participants (13 of 20) were clear of HSILs at their 24-month visit with response rates after the first, second, and third argon plasma coagulation treatments of 45, 44, and 67 percent, respectively. As with other treatment modalities, recurrences were common, and the study authors also highlight equipment cost as a potentially limiting feature[67].
Radiofrequency ablation (RFA) has been approved by the FDA for treatment of anal HSIL (and is the only treatment approved by the FDA for this indication). The technique provides circumferential ablation of the squamocolumnar junction and has been shown to be effective in small studies performed at a single center.68 Studies of larger numbers of patients in different centers need to be performed before this technique is widely adopted in the field.
For lesions that are too large for office-based local therapy, a combination of surgery and follow-up with office-based ablation may be effective[63].
The effectiveness and safety of this approach was illustrated by a retrospective review of the experience with 246 patients treated at the University of California San Francisco over a ten-year period[63]. Overall, 200 patients (81 percent) were treated with a single procedure, although 57 percent of these did develop recurrent disease at an average of 19 months after the initial procedure. In 46 patients (19 percent), multiple staged treatments were required for complete lesion ablation. Significant complications were observed in only nine patients (including one with bleeding requiring reoperation, two with anal stenosis, and four with anal fissures).
Similar results were observed in a series of 232 men treated with electrocautery in New York City[66]. The probability of disease control of the initial lesion after a single treatment was approximately 80 percent. Recurrent disease occurred in both HIV-uninfected and HIV-infected men (53 and 61 percent, respectively), but only one patient (0.4 percent) progressed to anal squamous cell carcinoma.
The high rate of local recurrence, even in patients initially thought to have been completely ablated with a single procedure, mandates careful surveillance following treatment[63]. Nonetheless; most such recurrences can be successfully managed with office-based therapy.
Only limited data are available comparing different treatment modalities in men with HSIL. In the only randomized trial, 156 men were randomly assigned to imiquimod, topical fluorouracil, or electrocautery. All grades of anal SIL were included; HSIL was present in 57 percent of cases. Patients with perianal SIL constituted 17 percent of the study[69].
Among the 148 patients actually treated (modified intent to treat), the complete response rates with imiquimod, fluorouracil, and electrocautery were 24, 17, and 39 percent, respectively. Recurrences were common, and by 72 weeks after initial treatment the cumulative recurrence rates were 71, 58, and 68 percent, respectively.
Due to high rates of recurrence and evolving AIN, surveillance is required following initial treatment. Although the optimal schedule has not been established, the approach is to follow up in four to six months, including a re-biopsy of the treatment site if there is lesion persistence. Anal cytology may also be useful as an adjunctive test to confirm lesion clearance.
References
- Douek DC, Roederer M, Koup RA. Emerging concepts in the immunopathogenesis of AIDS. Annu Rev Med 2009;60:471-484.
- Dandapani SV, Eaton M, Thomas CR, Pagnini PG. HIV- positive anal cancer: an update for the clinician. J Gastrointest Oncol. 2010;1:34-44.
- Davis KG, Orangio GR. Basic Science, Epidemiology, and Screening for Anal Intraepithelial Neoplasia and its Relationship to Anal Squamous Cell Cancer. Clin Colon Rectal Surg. 2018;31:368-378.
- SEER Cancer Statistics Factsheets: Anal Cancer. National Cancer Institute. Available from:http//seer.cancer.gov/statfacts/html/anus. html.
- Machalek DA, Poynten M, Jin F, Fairley CK, Farnsworth A, Garland SM, et al. Anal human papillomavirus infection and associated neoplastic lesions in men who have sex with men: a systematic review and meta-analysis. Lancet Oncol. 2012;13:487-500.
- Holly EA, Ralston ML, Darragh TM, Greenblatt RM, Jay N, Palefsky JM. Prevalence and risk factors for anal squamous intraepithelial lesions in women. J Natl Cancer Inst. 2001;93:843-849.
- adeleine MM, Finch JL, Lynch CF, Goodman MT, Engels EA. HPV-related cancers after solid organ transplantation in the United States. Am J Transplant. 2013;13:3202-3209.
- Chiao EY, Krown SE, Stier EA, Schrag D. A population-based analysis of temporal trends in the incidence of squamous anal canal cancer in relation to the HIV epidemic. JAIDS. 2005;40:451-455.
- Harden ME, Munger K. Human papilloma virus molecular biology. Mutat Res Rev Mutat Res. 2017 ;772:3-12.
- Herrero R, Gonzalez P, Markowitz LE. Present status of human papilloma virus vaccine development and implementation. Lancet Oncol 2015; 16: e20 6–e216.
- Teresa MD, Joel MP. Anal Cytology. In: Nayar R, Wilbur DC. (eds.).The Bethesda System for Reporting Cervical Cytology. Third edition. Switzerland: Springer International Publishing; 2015. p. 263-285.
- Daling JR, Madeleine MM, Johnson LG, Schwartz SM, Shera KA, Wurscher MA, et al. Human papillomavirus, smoking, and sexual practices in the etiology of anal cancer. Cancer. 2004;101:270-280.
- Lin CC, Hsieh MC, Hung HC, Tsao SM, Chen SC, Yang HJ, et al. Human papillomavirus prevalence and behavioral risk factors among HIV-infected and HIV-uninfected men who have sex with men in Taiwan. Medicine (Baltimore). 2018;97:e13201.
- Taylor S, Bunge E, Bakker M, Castellsagué X. The incidence, clearance and persistence of non-cervical human papillomavirus infections: a systematic review of the literature. BMC Infect Dis. 2016;16:293.
- Marchetti G, Comi L, Bini T, Rovati M, Bai F, Cassani B, et al. HPV Infection in a Cohort of HIV-Positive Men and Women: Prevalence of Oncogenic Genotypes and Predictors of Mucosal Damage at Genital and Oral Sites. J Sex Transm Dis. 2013;2013:915169.
- 14. Gandra S, Azar A, Wessolossky M. Anal high-risk human papillomavirus infection and high-grade anal intraepithelial neoplasia detected in women and heterosexual men infected with human immunodeficiency virus. HIV AIDS (Auckl) 2015;7:29–34.
- Lin CC, Hsieh MC, Hung HC, Tsao SM, Chen SC, Yang HJ, et al. Human papillomavirus prevalence and behavioral risk factors among HIV-infected and HIV-uninfected men who have sex with men in Taiwan. Medicine (Baltimore). 2018;97:e13201.
- Anderson J, Hoy J, Hillman R, Gittleson C, Hartel G, Medley G, et al. Abnormal anal cytology in high-risk human papilloma virus infection in HIV-infected Australians. Sex Transm Infect. 2008;84:94-96.
- Lee CH, Lee SH, Lee S, Cho H, Kim KH, Lee JE, et al. Anal Human Papillomavirus Infection among HIV-Infected Men in Korea. PLoS One. 2016;11:e0161460.
- Hernandez AL, Karthik R, Sivasubramanian M, Raghavendran A, Gnanamony M, Lensing S, et al. Prevalence of Anal HPV Infection Among HIV-Positive Men Who Have Sex With Men in India. J Acquir Immune DeficSyndr. 2016;71:437-443.
- Daling JR, Weiss NS, Hislop TG, Maden C, Coates RJ, Sherman KJ, et al. Sexual practices, sexually transmitted diseases, and the incidence of anal cancer. N Engl J Med. 1987;317:973-977.
- Qualters JR, Lee NC, Smith RA, Aubert RE. Breast and cervical cancer surveillance, United States, 1973–1987. MMWR CDC SurveillSumm. 1992;41:1–7.
- Ries L, Melbert D, Krapcho M, et al., editors. SEER SEaER. Age-adjusted SEER Incidence by site: Table I–4. National Cancer Institute; 2007.
- Chin-Hong P, Palefsky J. Natural history and clinical management of anal human papillomavirus disease in men and women infected with human immunodeficiency virus. Clin Infect Dis. 2002;35:1127–1134.
- Centers for Disease Control and Prevention. HPV-associated cancers statistics: HPV and cancer. Available from: http//www.cdc.gov/ cancer/hpv/statistics/cases.htm. Last accessed 13/12/2018.
- Gautier M, Brochard C, Lion A, Henno S, Mallet AL, Bodere A, et al. High-grade anal intraepithelial neoplasia: Progression to invasive cancer is not a certainty. Dig Liver Dis. 2016;48:806–811.
- Ogunbiyi OA, Scholefield JH, Raftery AT, Smith JH, Duffy S, Sharp F, et al. Prevalence of anal human papillomavirus infection and intraepithelial neoplasia in renal allograft recipients. Br J Surg. 1994;81:365–367.
- Ryan DP, Compton CC, Mayer RJ. Carcinoma of the anal canal. N Engl J Med. 2000. 342:792-800.
- Chiu S, Joseph K, Ghosh S, Cornand RM, Schiller D. Reasons for delays in diagnosis of anal cancer and the effect on patient satisfaction. Can Fam Physician. 2015. 61:e509-516.
- Möller C, Saksela E. Cancer of the anus and anal canal. Acta Chir Scand. 1970. 136:340-348.
- Hiorns LR, Scholefield JH, Palmer JG, Shepherd NA, Kerr IB. Ki-ras oncogene mutations in non-HPV-associated anal carcinoma. J Pathol. 1990.161:99-103.
- Daling JR, Madeleine MM, Johnson LG, Schwartz SM, Shera KA, Wurscher MA, et al. Human papillomavirus, smoking, and sexual practices in the etiology of anal cancer. Cancer. 2004. 101:270-280.
- Human papilloma virus (HPV) in patients with HIV 2018 guidelines. Available at https:// www.hivguidelines.org/sti-care/hpv-infection/. Last accessed on 23-11-2018.
- HIV/AIDS treatment guidelines. Available at https://aidsinfo.nih.gov/guidelines.Last accessed on 23-11-2018.
- British HIV Association guidelines for treatment of HIV-1 infected adults with anti retroviral therapy 2008. Available athttps:// www.bhiva.org/guidelines. Last accessed on 23-11-2018.
- Palefsky JM, Holly EA, Hogeboom CJ, Berry JM, Jay N, Darragh TM. Anal cytology as a screening tool for anal squamous intraepithelial lesions. J Acquir Immune DeficSyndr Hum Retrovirol. 1997;14:415-422.
- Scholefield JH, Johnson J, Hitchcock A, Kocjan G, Smith JH, Smith PA, et alGuidelines for anal cytology--to make cytological diagnosis and follow up much more reliable. Cytopathology. 1998;9:15-22.
- Bean SM, Chhieng DC. Anal-rectal cytology: a review. Diagn Cytopathol. 2010;38:538-546.
- Chiao EY, Giordano TP, Palefsky JM, Tyring S, El Serag H. ScreeningHIV-infected individuals for anal cancer precursor lesions: a systematic review. Clin Infect Dis. 2006 ;43: 223-233.
- Lindsey K, DeCristofaro C, James J. Anal Pap smears: Should we be doing them? J Am Acad Nurse Pract. 2009;21: 437-443.
- Moore HG, Guillem JG. Anal neoplasms. Surg Clin North Am. 2002;82:1233-1251.
- Richel O, Hallensleben ND, Kreuter A, van Noesel CJ, Prins JM, de Vries HJ. High-resolution anoscopy: clinical features of anal intraepithelial neoplasia in HIV-positive men. Dis Colon Rectum. 2013;56:1237–1242.
- Jay N, Berry JM, Hogeboom CJ, Holly EA, Darragh TM, Palefsky JM. Colposcopic appearance of anal squamous intraepithelial lesions: relationship to histopathology. Dis Colon Rectum. 1997;40:919–928.
- Camus M, Lesage AC, Fléjou JF, Hoyeau N, Atienza P, Etienney I. Which lesions should be biopsied during high-resolution anoscopy? Prospective descriptive study of simple morphological criteria. J Low Genit Tract Dis. 2015;19:156–160
- Kreuter A, Potthoff A, Brockmeyer NH, Gambichler T, Swoboda J, Stücker M, et al. Analcarcinoma in human immunodeficiency virus-positive men:results of a prospective study from Germany. Br J Dermatol. 2010;162:1269-1277.
- Fauci AS, Folkers GK, Lane HC. Human Immunodeficiency Virus Disease: AIDS and Related Disorders. In: Jameson JL, Kasper DL, Longo DL, Fauci AS, Hauser SL, Loscalzo J.(eds.). Harrison’s Principles of Internal Medicine. 20th edition. New York: McGraw-Hill Education; 2018. p. 1451
- Goldie SJ, Kuntz KM, Weinstein MC, Freedberg KA, Palefsky JM. Cost-effectiveness of screening for anal squamous intraepithelial lesions and anal cancer in human immunodeficiency virus-negative homosexual and bisexual men. Am J Med 2000; 108:634.
- Goldie SJ, Kuntz KM, Weinstein MC, Freedberg KA, Welton ML, Palefsky JM. The clinical effectiveness and cost-effectiveness of screening for anal squamous intraepithelial lesions in homosexual and bisexual HIV-positive men. JAMA. 1999;281:1822.
- Kim DK, Riley LE, Hunter P. Advisory Committee on Immunization Practices Recommended Immunization Schedule for Adults Aged 19 Years or Older - United States, 2018. MMWR Morb Mortal Wkly Rep 2018; 67:158.
- Markowitz LE, Dunne EF, Saraiya M, et al. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2014; 63:1.
- Petrosky E, Bocchini JA Jr, Hariri S, et al. Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep 2015; 64:300.
- Robinson CL, Romero JR, Kempe A, et al. Advisory Committee on Immunization Practices Recommended Immunization Schedule for Children and Adolescents Aged 18 Years or Younger - United States, 2018. MMWR Morb Mortal Wkly Rep 2018; 67:156.
- Giuliano AR, Palefsky JM, Goldstone S, et al. Efficacy of quadrivalent HPV vaccine against HPV Infection and disease in males. N Engl J Med 2011; 364:401.
- Palefsky JM, Giuliano AR, Goldstone S, Moreira ED, Aranda C, Jessen H, Hillman R, Ferris D, Coutlee F, Stoler MH, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med. 2011;365:1576–1585.
- Joura EA, Giuliano AR, Iversen OE, Bouchard C, Mao C, Mehlsen J, Moreira ED, Ngan Y, Petersen LK, Lazcano-Ponce E, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372:711–723.
- Cranston RD, Baker JR, Liu Y, et al. Topical application of trichloroacetic acid is efficacious for the treatment of internal anal high-grade squamous intraepithelial lesions in HIV-positive men. Sex Transm Dis 2014; 41:420.
- Singh JC, Kuohung V, Palefsky JM. Efficacy of trichloroacetic acid in the treatment of anal intraepithelial neoplasia in HIV-positive and HIV-negative men who have sex with men. J Acquir Immune DeficSyndr 2009; 52:474.
- Richel O, Wieland U, de Vries HJ, et al. Topical 5-fluorouracil treatment of anal intraepithelial neoplasia in human immunodeficiency virus-positive men. Br J Dermatol 2010; 163:1301.
- Kreuter A, Potthoff A, Brockmeyer NH, et al. Imiquimod leads to a decrease of human papillomavirus DNA and to a sustained clearance of anal intraepithelial neoplasia in HIV-infected men. J Invest Dermatol 2008; 128:2078.
- Fox PA, Nathan M, Francis N, et al. A double-blind, randomized controlled trial of the use of imiquimod cream for the treatment of anal canal high-grade anal intraepithelial neoplasia in HIV-positive MSM on HAART, with long-term follow-up data including the use of open-label imiquimod. AIDS 2010; 24:2331.
- Halasz CL. Treatment of common warts using the infrared coagulator. J Dermatol Surg Oncol 1994; 20:252.
- Cranston RD, Hirschowitz SL, Cortina G, Moe AA. A retrospective clinical study of the treatment of high-grade anal dysplasia by infrared coagulation in a population of HIV-positive men who have sex with men. Int J STD AIDS 2008; 19:118.
- Pineda CE, Berry JM, Jay N, et al. High-resolution anoscopy targeted surgical destruction of anal high-grade squamous intraepithelial lesions: a ten-year experience. Dis Colon Rectum 2008; 51:829.
- Weis SE, Vecino I, Pogoda JM, Susa JS. Treatment of high-grade anal intraepithelial neoplasia with infrared coagulation in a primary care population of HIV-infected men and women. Dis Colon Rectum 2012; 55:1236.
- Goldstone RN, Goldstone AB, Russ J, Goldstone SE. Long-term follow-up of infrared coagulator ablation of anal high-grade dysplasia in men who have sex with men. Dis Colon Rectum 2011; 54:1284.
- Marks DK, Goldstone SE. Electrocautery ablation of high-grade anal squamous intraepithelial lesions in HIV-negative and HIV-positive men who have sex with men. J Acquir Immune DeficSyndr 2012; 59:259.
- de Pokomandy A, Rouleau D, Lalonde R, et al. Argon plasma coagulation treatment of anal high-grade squamous intraepithelial lesions in men who have sex with men living with HIV: results of a 2-year prospective pilot study. HIV Med 2018; 19:81.
- Goldstone RN, Hasan SR, Goldstone SE. Brief Report: Radiofrequency Ablation Therapy for Anal Intraepithelial Neoplasia: Results From a Single-Center Prospective Pilot Study in HIV+ Participants. J Acquir Immune DeficSyndr 2017; 76:e93.
- Richel O, de Vries HJ, van Noesel CJ, et al. Comparison of imiquimod, topical fluorouracil, and electrocautery for the treatment of anal intraepithelial neoplasia in HIV-positive men who have sex with men: an open-label, randomised controlled trial. Lancet Oncol 2013; 14:346.