Information
Journal Policies
Five-Year Visual Outcome and Visual Prognostic Factors after Photodynamic Therapy with Verteporfin for Idiopathic Choroidal Neovascularization
Hae Min Kang, MD1, 2, Hyoung Jun Koh, MD2
2Institute of Vision Research, Department of Ophthalmology, Yonsei University College of Medicine, Seoul, Republic of Korea
Copyright : © 2016 Hae MK. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Aim: To evaluate the long-term outcome of photodynamic therapy (PDT) for idiopathic choroidal neovascularization (CNV).
Methods: Retrospective review of 30 eyes (27 patients) treated with PDT for naïve idiopathic CNV, and completed at least five-year follow-up. Best-corrected visual acuity (BCVA, logMAR; logarithm of minimum angle resolution) at baseline and each follow-up visit were investigated. Baseline characteristics including greatest linear diameter at baseline (mm), location of CNV (juxtafoveal or subfoveal), refractive errors, age at diagnosis, and presence of recurrence were analyzed.
Results: During mean follow-up of 76.0±15.5 months, mean total 1.87±1.31 numbers of PDT were done. Mean age at diagnosis of idiopathic CNV was 36.7±6.2 years. Eight eyes (26.7%) showed 1.3±0.7 recurrences. Nineteen eyes (63.3%) showed subfoveal CNV, and 11 eyes (36.7%) did juxtafoveal CNV. Mean BCVA at baseline was 0.56±0.38 logMAR (20/72 Snellen equivalent), and 0.56±0.86 logMAR at 5 years (20/72 Snellen equivalent; p=0.980). Baseline BCVA (B=-7.250, P=0.015), juxtafoveal location of CNV (B=0.825, P=0.038), and baseline greatest linear diameter (B=-0.835, P=0.047) were significantly correlated with visual outcome.
Conclusion: Five-year follow-up results of PDT for idiopathic CNV showed limited efficacy in visual outcome. Baseline BCVA, lesion size, and location of CNV were significantly correlated with long-term visual outcome after PDT.
Keywords: Choroidal neovascularization; Idiopathic choroidal neovascularization; Photodynamic therapy
1.Introduction
Idiopathic choroidal neovascularization (CNV) is defined as CNV in patients younger than 50 years, without any apparent primary ocular or systemic diseases.[1,2] The natural history and final visual outcomes of idiopathic CNV are generally considered to be more favorable than CNV due to age-related macular degeneration (AMD) [3,4]. However, the natural course of the disorder can be unpredictable, and treatment is recommended for these patients [1,3,4]
Various treatment modalities have been used for idiopathic CNV. Because idiopathic CNV usually appears as classic CNV, photodynamic therapy (PDT) was recommended as mainstay of treatment for idiopathic CNV before the introduction of anti-vascular endothelial growth factor (VEGF) agents [5, 6]. Even in the era of anti-VEGF, PDT is still considered a good treatment option for some diseases, including polypoidal choroidal vasculopathy (PCV) [7, 8] A recent retrospective study demonstrated favorable visual outcomes after PDT for PCV with long-term follow-up periods over 60 months [9]. Long-term follow-up study for myopic CNV also showed a stabilizing effect of PDT, suggesting PDT as an attractive option, especially for juxtafoveal myopic CNV [10].
Long-term treatment efficacy of PDT has not been investigated for idiopathic CNV, although it was considered as a good treatment option for idoipathic CNV. In this study, we investigated the 5-year visual outcomes of eyes with idiopathic CNV after PDT, to determine the long-term efficacy and safety of PDT. In addition, additional analysis was performed to find out visual prognostic factors after PDT in these patients.
2.Methods
The medical records for 30 eyes of 27 patients with naive idiopathic CNV were retrospectively reviewed. The first treatment of each patient was performed between March 2005 and March 2007, and all included patients completed at least 5 years of follow-up at Yonsei University Medical Center. Informed consent was obtained from all eligible participants at the time of treatment, and this study was approved by the Institutional Review Board of Yonsei University Medical Center.
Inclusion criteria for idiopathic CNV were: (1) age <50 years; and (2) absence of concurrent ocular diseases in the study eyes that compromised or could have compromised vision, such as pathologic myopia.. Exclusion criteria were: (1) history of prior treatment for CNV (including laser, submacular surgery, PDT, or intravitreal anti-VEGF therapy); (2) extrafoveal location of CNV.
The patients’ characteristics were retrieved from the medical charts, including age at initial diagnosis, gender, refractive errors, greatest linear diameter at baseline, and best-corrected visual acuity (BCVA) determined using Decimal charts.. Decimal BCVA results were converted to a logarithm of the minimum angle of resolution (logMAR) value for statistical analysis. The refractive errors for all patients were measured by an autorefractometer without cycloplegia. We further divided the patients into two groups according to the location of CNV lesion: juxtafovea and subfovea, and compared visual outcome between two groups.Greatest linear dimension at baseline was measured manually at the early phase of ICGA, using software embeeded in the Heidelberg Retinal Angiograph system (HRA-2; Heidelberg engineering, Dossenheim, Germany).
PDT with verteporfin was performed according to previous studies.[11,12] We defined the recurrence of idiopathic CNV as the reappearance of active CNV lesions with leakage on FA after more than 6 months without treatment. Retreatment for recurrence was considered based upon FA, ICGA, and optical coherence tomography findings.
The patients were observed 1 month after PDT, and at 1- to 2-month intervals based on the patients' condition during the first year of treatment. When there was no evidence of active disease, annual follow-up was done for the patients. At every visit, BCVA testing, slit lamp examination, and dilated fundus examination were performed. In cases of changing symptoms, including vision worsening or metamorphopsia, additional FA, ICGA, and optical coherence tomography were performed to rule out recurrence of CNV.
The primary measured outcomes were the mean BCVA from baseline at each follow-up. We also defined visual gain as 0.3 logMAR or more visual improvement, and visual loss as visual deterioration of 0.3 logMAR or more when compared with baseline.
The Kolmogorov-Smirnove test was used for verification of normal distribution in this study group. Paired t-test was used to compare the baseline BCVA to that at each visit. In addition, non-parametric analyses including the Mann-Whitney U test and Wilcoxon signed-rank test were used for subgroup analyses. Stepwise multiple regression analysis was performed to find out predictive factors for visual outcome. Statistical analysis was performed using SPSS 18.0 software for Windows (SPSS Inc., Chicago, IL, USA). P values of less than 0.05 were considered statistically significant.
3.Results
Mean age at diagnosis was 36.73±6.15 years, and 19 patients (63.3%) were female. All eyes showed type 2 CNV by FA, and distinct dark rims surrounding CNV lesions by ICGA. The greatest linear diameter was 947.16±484.95 µm at baseline. During mean follow-up of 75.97±15.50 months, a total mean number of PDT was 1.87±1.31 times.. 8 eyes (26.7%) experienced at least one recurrence, with mean number of recurrence as 1.33±0.71 times. The mean interval between first remission and first recurrence was 21.57±2.37 months. Mean number of PDT treatments was 2.89±1.62 times in the eyes with recurrence. Patients’ characteristics were summarized in Table 1.
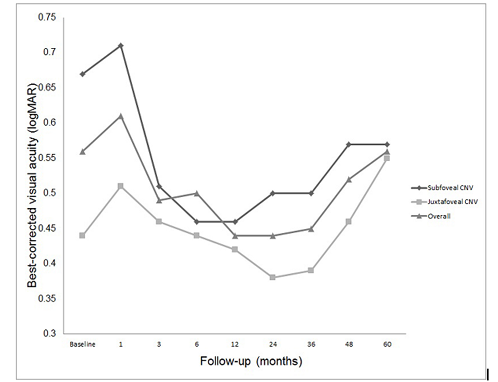
Mean BCVA was 0.56±0.38 logMAR (20/72 Snellen equivalent) at baseline, and 0.56±0.86 logMAR (20/72 Snellen equivalent) at 5 years (P=0.980). Changes of mean BCVA during follow-up are depicted in Figure 1. There was no significant difference between mean BCVA at each follow-up and baseline (P=0.581 at 1 month, P=0.576 at 3 months, P=0.681 at 6 months, P=0.417 at 1 year, P=0.452 at 2 years, P=0.480 at 3 years, and P=0.822 at 4 years).
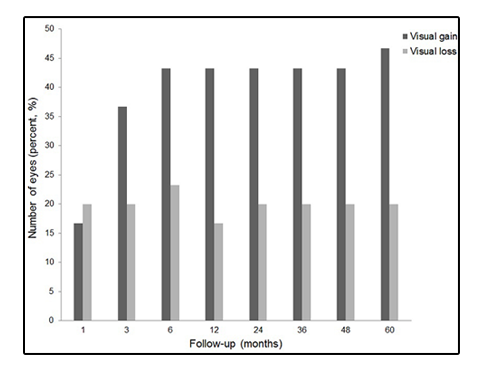
BCVA at each follow-up were classified as visual gain and deterioration when compared with baseline BCVA (Figure 2). 14 eyes (46.7%) gained vision, whereas 6 eyes (20.0%) lost vision at 5-year follow-up visits. The 6 eyes with visual loss at 5 years showed disciform scarring at the fovea.
Figure1. Changes of mean best-corrected visual acuity (BCVA; logarithm of the minimum angle of resolution, logMAR) from baseline and each follow-up in eyes with idiopathic choroidal neovascularization (CNV) after photodynamic therapy. There was no significant change at each follow-up visit when compared with baseline.
Figure2. Number of eyes showing visual gain and visual loss after photodynamic therapy for idiopathic choroidal neovascularization. Visual gain was defined as improvement of best-corrected visual acuity (BCVA) 0.3 logarithm of the minimum angle resolution (logMAR) or more, and visual loss was defined as deterioration of BCVA 0.3 logMAR or more than baseline BCVA. Among overall patients, 14 eyes (46.7%) gained vision, whereas 6 eyes (20.0%) lost vision at 5 years.
Mean BCVA at baseline was 0.67±0.39 logMAR (20/93 Snellen equivalent) in subfoveal group, and 0.44± 0.36 logMAR (20/55 Snellen equivalent) in juxtafoveal group (P=0.173). Mean BCVA at 5 years was 0.57± 0.91 logMAR in subfoveal group, and 0.55± 0.86 logMAR in juxtafoveal group (P=0.705). The changes of mean BCVA in each group were depicted in Figure 1.
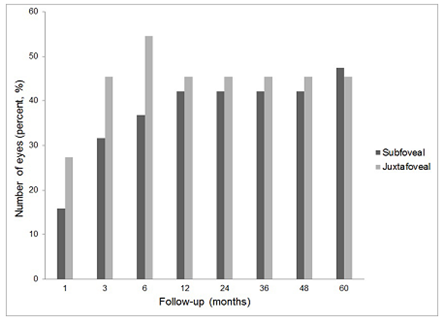
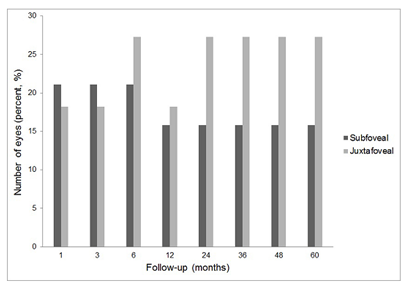
The eyes of subfoveal and juxtafoveal groups were also classified into visual gain and visual deterioration according to the BCVA changes from baseline BCVA. At 5 years, 9 eyes (47.4%) of the subfoveal group and 5 eyes (45.4%) of the juxtafoveal group gained vision (Figure 3). 3 eyes of each group (subfoveal, 15.8%; and juxtafoveal, 27.3%) lost vision at 5 years (Figure 4). Representative case was shown in Figure 5.
Figure3. Percentage of eyes with visual gain defined as visual improvement of 0.3 logarithm of the minimum angle resolution (logMAR) or more when compared with baseline after photodynamic therapy for idiopathic choroidal neovascularization (CNV). Among the eyes with subfoveal CNV, 47.4% (9 eyes) gained vision, and 45.4% (5 eyes) with juxtafoveal CNV gained vision at 5 years when compared with baseline.
Figure4. Percentage of eyes with visual loss defined as visual deterioration 0.3 logarithm of the minimum angle resolution (logMAR) or more when compared with baseline after photodynamic therapy for idiopathic choroidal neovascularization (CNV). 15.8% (3 eyes) with subfoveal CNV, and 27.3% (3 eyes) with juxtafoveal CNV lost vision when compared with baseline.
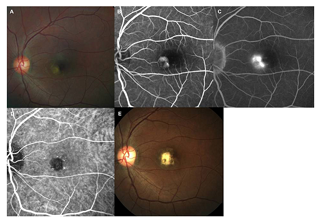
Figure5. A 40-year-old female patient visited the ophthalmology clinic, complaining decreased vision in the left eye for 1 month. Best-corrected visual acuity (BCVA) of the left eye was 0.4 logarithm of the minimum angle resolution (logMAR). The greatest linear diameter was 1.25 mm at baseline. Refractive error with spherical equivalent was -1.50 diopter in both eyes. (Top left) On fundus examination, well-demarcated subretinal gray membrane with exudative fluid was shown at juxtafovea. Otherwise, there was no remarkable findings in fundus of the left eye. (Top middle) Well demarcated juxtafoveal choroidal neovascularization was documented on the early phase fluorescein angiography (FA). (Top right) Late phase FA showed profuse leakage from neovascular membrane. (Bottm left) Well demarcated neovascular membrane was surrounded with dark rim on indocyanine green angiography. Conventional photodynamic therapy was performed involving the entire neovascular membrane. (Bottom right) 5 years after photodynamic therapy, fundus examination showed subretinal fibrosis involving fovea. BCVA of the left eye was count finger.
None of the patients developed systemic or ocular complications associated with PDT or supplemental verteporfin.
4.Discussion
In this study, we evaluated the long-term efficacy of PDT for idiopathic CNV. At the end of follow-up, 46.7% gained vision, whereas 20.0% lost vision when compared with baseline. The results of current study indicated that PDT seemed to have a long-term stabilizing efficacy for idiopathic CNV. However, when compared with a natural history study which showed only 5% of eyes with significant visuasl loss,[1] long-term follow-up of PDT showed limited visual outcome. When classified the eyes into subfoveal and juxtafoveal, those with juxtafoveal CNV lost gain more than those with subfoveal CNV, despite of better baselilne BCVA. The results suggest that those with juxtafoveal CNV showed worse visual outcome when treated with PDT.
Limited long-term visual outcome, especially with those with juxtafoveal CNV, may be due to harmful effect of PDT on retinal pigment epithelium (RPE). Dark rim surrounding idiopathic CNV on ICGA corresponds to multilayered and proliferated RPE at the outer margin of the neovascular membrane [13-15] This proliferated RPE layer seems to be related to the recovery mechanism of RPE which suppresses neovascularization [16,17] However, PDT can compromise this recovery mechanism of RPE, leading to limited recovery in these patients. It has been well documented that standard PDT could induce various changes including RPE damage [18]. Various degrees of RPE damage and RPE atrophy following PDT also have been reported [19,20]. These deteriorating effects of PDT on RPE may lead to a limited long-term visual outcome in idiopathic CNV, especially in juxtafoveal CNV in this study.
We also investigated the prognostic factors for long-term visual outcome after PDT in idiopathic CNV. Results showed that baseline BCVA, initial lesion size (greatest linear diameter at baseline), and location of CNV were visual prognostic factors for visual outcome. It has been well documented that baseline lesion size and BCVA were significantly correlated with visual outcome in AMD patients,including PCV [21-23]. In addition, the results of our study showed that juxtafoveal location of CNV was associated with worse visual outcome than subfoveal CNV after PDT. This may be due to the harmful effect of PDT on foveal center in the patients with juxtafoveal CNV, with further compromising foveal function.
This study has several limitations, including its retrospective nature and the relatively small study population. In addition, anti-VEGF therapy has become the mainstay of treatment for idiopathic CNV [24,25]. When compared with PDT, anti-VEGF therapy showed better visual outcome [26]. However, we think validation of long-term visual outcome after PDT is also important in idiopathic CNV. Our results indirectly support the use of anti-VEGF therapy, and can be used as a control data when validating the long-term efficacy of anti-VEGF therapy.
In conclusion, PDT for idiopathic CNV showed limited long-term visual outcome. Baseline BCVA, initial lesion size, and location of CNV were significantly correlated visual long-term visual outcome in these patients.
4.Conclusion
RDW is a sensitive (79.46%) indicator in detecting or screening IDA thus aiding in early diagnosis of the children. So, RDW of more than 17.9% can be used as an effective tool for the diagnosis of IDA in a large number of samples at major hospitals thereby reducing the manpower and unnecessary time consumption.
5.Acknowledgement
a. Funding/Support: none
b. Financial Disclosure: none
c. Contributions of Authors: Design and conduct of study (HM Kang, HJ Koh); collection of data (HM Kang); Management, analysis, and interpretation of data (HM Kang, HJ Koh); and preparation (HM Kang, HJ Koh); review, and approval of the manuscript (HM Kang, HJ Koh)
d. Other Acknowledgments: none
6.References
- Ho AC, Yannuzzi LA, Pisicano K, and De Rosa J. The natural history of idiopathic subfoveal choroidal neovascularization. Ophthalmology 102(5), Pp 782-789 (1995)
- Lempert P. Idiopathic subfoveal choroidal neovascularization. Ophthalmology 102(10), Pp 1411-1412 (1995)
- Macular Photocoagulation Study Group. Krypton laser photocoagulation for idiopathic neovascular lesions. Results of a randomized clinical trial. Arch Ophthalmol 108(6), Pp 832-837 (1990)
- Macular Photocoagulation Study Group. Argon laser photocoagulation for idiopathic neovascularization. Results of a randomized clinical trial. Arch Ophthalmol 101(9), Pp 1358-1361 (1983)
- Chan WM, Lam DS, Wong TH, Lai TY, Kwok AK, Tam BS, and Li KK. Photodynamic therapy with verteporfin for subfoveal idiopathic choroidal neovascularizatioin: one-year results from a prospective case series. Ophthalmology 110(12), Pp 2395-2402 (2003)
- Spaide RF, Martin ML, Slakter J, Yannuzzi LA, Sorenson J, Guyer DR, and Freund KB. Treatment of idiopathic subfoveal choroidal neovascular lesions using photodynamic therapy with verteporfin. Am J Ophthalmol 134(1), Pp 62-68 (2002)
- Gomi F, Ohji M, Sayanagi K, Sawa M, Sakaguchi H, Oshima Y, Ikuno Y, and Tano Y. One-year outcomes of photodynamic therapy in age-related macular degeneration and polypoidal choroidal vasculoopathy in Japanese patients. Ophthalmology 115(1), Pp 141-146 (2008)
- Chan WM, Lam DS, Lai TY, Liu DT, Li KK, Yao Y, and Wong TH. Photodynamic therapy with verteporfin for symptomatic polypoidal choroidal vasculopathy. Ophthalmology 111(8), Pp 1576-1584 (2004)
- Kang HM, Kim YM, and Koh HJ. Five-year follow-up results of photodynamic therapy for polypoidal choroidal vasculopathy. Am J Ophthalmol 155(3), Pp 438-447 (2013)
- Hayashi K, Ohno-Matsui K, Shimada N, Moriyama M, Hayashi W, Wang J, Yoshida T, Tokoro T, and Mochizuki M. Long-term results of photodynamic therapy for choroidal neovascularization in Japanese patients with pathologic myopia. Am J Ophthalmol 151(1), Pp 137-147 (2011)
- Treatment of Age-related macular degeneration with Photodynamic therapy (TAP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: one-year results of 2 randomized clinical trials-TAP report. Arch Ophthalmol 117(10), Pp 1329-1345 (1999)
- Verteporfin in Photodynamic Therapy Study Group. Verteporfin therapy of subfoveal choroidal neovascularization in age-related macular degeneration: two-year results of a randomized clinical trial including lesions with occult with no classic choroidal neovascularizatioin-Verteporfin in Photodynamic Therapy Report 2. Am J Ophthalmol 131(5), Pp 541-560 (2001)
- Iida T, Hagimura N, Kish S, and Shimizu K. Indocyanine green angiographic features of idiopathic submacular neovascularization. Am J Ophthalmol 126(1), Pp 70-76 (1998)
- Grossniklaus HE, and Gass JDM. Clinicopathologic correlations of surgically excised type 1 and type 2 submacular choroidal neovascular membranes. Am J Ophthalmol 126(1), Pp 59-69 (1998)
- Grossniklaus HE, Hutchinson AK, Capone A, Woolfson J, and Labert HM. Clinicopathologic features of surgically excised choroidal vascular membranes. Ophthalmology 101(6), Pp 1099-1111 (1994)
- Miller H, Miller B, and Ryan SJ. The role of retinal pigment epithelium in the involution of subretinal neovascularization. Invest Ophthalmol Vis Sci 27(11), Pp 1644-1652 (1986)
- Glaser BM, Campochiaro PA, Davis JL Jr, and Jerdan JA. Retinal pigment epithelial cells release inhibitors of neovascularization. Ophthalmology 94(7), Pp 780-784 (1987)
- Tzekov R, Lin T, Zhang KM, Jackson B, Oyejide A, Orilla W, Kulkarni AD, Kuppermann BD, Wheeler L, and Burke J. Ocular changes after photodynamic therapy. Invest Ophthalmol Vis Sci 47(1), Pp 377-385 (2006)
- Postelmans L, Pasteels B, Coquelet P, El Ouardighi H, Verougstraete C, and Schmidt-Erfurth U. Severe pigment epithelial alterations in the treatment area following photodynamic therapy for classic choroidal neovascularization in young females. Am J Ophthalmol 138(5), Pp 803-808 (2004)
- Sii F, and Lee LR. Retinopathy associated with photodynamic therapy for treatment of idiopathic choroidal neovascularization. Clin Experiment Ophthalmol 34(2), Pp 184-186 (2006)
- Yamashiro K, Tomita K, Tsujikawa A, Nakata I, Akagi-Kurashige Y, Miyake M, Ooto S, Tamura H, and Yoshimura N. Factors associated with the response of age-related macular degeneration to intravitreal ranibizumab treatment. Am J Ophthalmol 154(1), Pp 125-136 (2012)
- Axer-Siegel R, Ehrlich R, Yassur Y, Rosenblatt I, Kramer M, Priel E, Benjamini Y, and Weinberger D. Photodynamic therapy for age-related macular degeneration in a clinical setting: visual results and angiographic patterns. Am J Ophthalmol 137(2), Pp 258-264 (2004)
- Hikichi T, Ohtsuka H, Higuchi M, Matsushita T, Ariga H, Kosaka S, Matsushita R, and Takami K. Factors predictive of visual acuity outcomes 1 year after photodynamic therapy in Japanese patients with polypoidal choroidal vasculopathy. Retina 31(5), Pp 857-865 (2011)
- Zhang H, Liu ZL, and Gu F. Intravitreal bevacizumab for treatment of subfoveal idiopathic choroidal neovascularization: results of a 1-year prospective trial. Am J Ophthalmol 153(2), Pp 300-306 (2012)
- Mandal S, Garg S, Venkatesh P, Mithal C, Vohra R, and Mehrotra A. Intravitreal bevacizumab for subfoveal idiopathic choroidal neovascularization. Arch Ophthalmol 125(11), Pp 1487-1492 (2007)
- Kang HM, and Koh HJ. Intravitreal anti-vascular endothelial growth factor therapy versus photodynamic therapy for idiopathic choroidal neovascularization. Am J Ophthalmol 155(4), Pp 713-719 (2013)